Management of lower Gastrointestinal malignancies
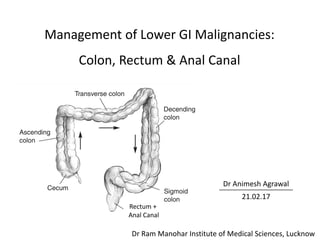
- 1. Management of Lower GI Malignancies: Colon, Rectum & Anal Canal Dr Animesh Agrawal 21.02.17 Rectum + Anal Canal Dr Ram Manohar Institute of Medical Sciences, Lucknow
- 2. • Management options • Stage wise management • Radiotherapy techniques • Follow up
- 3. Carcinoma of the Colon
- 4. Tx Tumor cannot be assessed T0 No evidenceof priary tumor Tis Carcinoma in situ: intraepithelial or invasion of lamina propria T1 Tumor invades submucosa T2 Tumor invades muscularis propria T3 Tumor invades pericolorectal tissues T4a Tumor penetrates to the surface of the visceral peritoneum T4b Tumor directly invades or is adherent to other organs or structure Nx Regional lymph nodes cannot be assessed N0 No regional lymph node metastasis N1 Metastasis in 1–3 regional lymph nodes N1a Metastasis in one regional lymph node N1b Metastasis in 2-3 regional lymph nodes N1c Tumor deposit(s) in the subserosa, mesentery, or nonperitonealized pericolic or perirectal tissues without regional nodal metastasis N2a Metastasis in 4–6 regional lymph nodes N2b Metastasis in > 7regional lymph nodes
- 5. M0 No distant metastasis M1 Distant metastasis M1a Metastasis confi ned to one organ or site (e.g., liver, lung, ovary, nonregional node) M1b Metastases in more than one organ/site or the peritoneum Changes in the 8th edition; applicable from 2018 M0 No distant metastasis M1 Distant metastasis M1a Metastasis confined to one organ or site (e.g., liver, lung, ovary, nonregional node) without peritoneal metastases M1b Metastases in more than one organ/site or the peritoneum. M1c Metastasis to the peritoneum with or without other organ involvement. AJCC/UICC Staging System; 7th edition
- 6. Management depends on Multimodality treatment • Surgery • Chemotherapy • Targeted therapy • Radiotherapy Tumor related • Stage • Location Patient related • Age • Performance status • Medical comorbidities • Molecular markers Treatment options
- 7. Surgery • Colectomy - Principal treatment modality - Accurate disease staging - Guides adjuvant treatment • Intent: - Curative - Palliative • Indications: - Stage 1 – 3 - Resectable stage 4
- 8. Aim of surgery • Primary objective is an R0 resection: Considered curative • Wide resection of involved colon segment + Lymphatics + Mesocolon + en-bloc resection of neighbouring organs with adequate margin f/b reconstituting bowel continuity • Margin ~5 cm of normal bowel proximal and distal to tumor considered adequate • Minimum of 12 nodes removed, suspicious nodes outside field of resection removed or biopsied. • Positive LN left behind considered R2 resection. • Inspect abdomen viscera, peritoneum, non-localized lymph nodes for mets. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst 2001; 93:583.
- 9. • Right/left Hemicolectomy • Extended Right/left Hemicolectomy • Transverse colectomy • Segmental resection • Total Abdominal Colectomy: UC, FAP Syndrome Types of Colectomy
- 10. Extent of colectomy • Dictated by the size and location of lesions, vascular and lymphatic supply. • Lymphatics of the intraperitoneal colon are found within the mesentery of the bowel.
- 12. D B CA E F A. Rt Hemicolectomy B. Rt Extended hemicolectomy C. Transverse colectomy D & E. Lt Hemicolectomy F. Sigmoid Resection
- 13. Laparoscopic-assisted colectomy • May be considered based upon the following criteria: - Experience surgeon performing laparoscopically assisted colorectal operations. - No locally advanced disease. - Not indicated for acute bowel obstruction or perforation. • Thorough abdominal exploration to be done. NCCN 2017
- 14. Laparoscopic vs Open colectomy • Randomized/non randomized prospective study data suggest no difference in oncologic outcome in open vs. laparoscopic resection.(1-5) Advantages of Disadvantages • Good abdominal exploration & visualization of visceral structures • Small incision • Early return of bowel function • Faster recovery& less hospital stay • Inadequacy of resection margin • Nodal sampling is difficult • Risk of seeding port sites • Cost 1. COST Study Group, NEJM 2004 2. Fleshman et al, Ann Surg 2007 3. Bonjer et al, Arch Surg 2007 3. Jackson et al, J Am Coll Surg 2007 4. CLASSIC study group. 2005, 2013
- 15. Pathologic report Parameters to be reported: • Depth • Number of L.N evaluated • Number of L.N positive • Status of margin: proximal, distal, and radial • Grade • LVI • PNI • Extranodal tumor deposits College of American Pathologists Consensus Statement; NCCN 2017
- 16. CAP Statement on Prognostic Factors • Category I (factors definitively proven to be of prognostic importance) 1. Local extent of tumor assessed pathologically (pT stage). 2. Regional lymph node metastasis (the pN stage) 3. LVI 4. Residual tumor following surgery with curative intent 5. Preoperative elevation of CEA • Category IIA (Factors studied extensively for prognostication, but not validated prospectively) 1. Tumor grade 2. Radial margin status (for resection specimens with nonperitonealized surfaces) 3. Residual tumor in the resection specimen following neoadjuvant therapy • Category IIB (Factors studied for association, not fitting in I or IIA) 1. Histologic type 2. Histologic features associated with microsatellite instability (MSI) (ie, host lymphoid response to tumor and medullary or mucinous histologic type) 3. High degree of MSI (MSI-H) 4. Loss of heterozygosity at 18q (DCC gene allelic loss) 5. Tumor border configuration (infiltrating vs pushing border) Compton et al. Arch Pathol Lab Med 2000
- 17. • Category III (Factors not studied sufficiently to know prognostic value) 1. DNA content 2. All other molecular markers except loss of heterozygosity 18q/DCC and MSI-H 3. PNI 4. Microvessel density 5. Tumor cell–associated proteins or carbohydrates 6. Peritumoral fibrosis 7. Peritumoral inflammatory response 8. Focal neuroendocrine differentiation 9. Nuclear organizing regions 10. Proliferation indices. • Category IV (Factors well studied; no importance) 1. Tumor size 2. Gross tumor configuration. Compton et al. Arch Pathol Lab Med 2000 CAP Statement on Prognostic Factors
- 18. Results of surgical resection • Resection results in excellent cure rates for lesions limited to the bowel wall with negative nodes (T1-T2 N0) • With a single high-risk feature - extension beyond colonic wall (T3+) or involved nodes (N+), 5-year survival with surgery falls below 75%, and adjuvant treatment may be indicated. • When both high-risk features (T3–4 N+), 5-year survival with surgery alone drops to approximately 50% (T3 N+) and 35% (T4 N+), and adjuvant treatment is recommended. Stage Mean 5 yr survival (%) T1N0 97 T2N0 85-90 T3N0 78 T4N0 63 T2N+ 74 T3N+ 48 T4N+ 35
- 19. Survival by N stage in various T stages; by number of nodes examined. (Colon cancer, 5 yr OS: SEER database) A-E: T1-T4b (Each curve represents an N stage)
- 20. Adjuvant Chemotherapy In Resected Colon Cancer
- 21. • Stage I: No adjuvant treatment • Stage II • Role of adjuvant chemotherapy unclear. • Risk estimation - pT4 - Grade 3-4 - LVI + - PNI + - < 12 nodes examined - Indeterminate, close or + margin - obstruction, perforation NCCN 2017
- 22. Stage II (contd) May consider for high Risk IIA & Stage IIB, IIC • FOLFOX • mFOLFOX6 • Cape-Ox • FLOX Low Risk IIA • Observation • Clinical trial. • May consider single agent Capecitabine or 5FU + leucovorin. NCCN 2017
- 23. Stage III Role of adjuvant chemotherapy established Preferred • FOLFOX • CapeOx Other options • mFOLFOX6 • FLOX May consider • Capecitabine • 5FU + LV NCCN 2017
- 24. Evolution of chemotherapy regimen for Ca Colon 1. 5FU + Levamisole 2. 5FU + Leucovorin 3. oral 5 FU analogue 4. Addition of oxaliplatin to 5FU/LV 5. Addition of irinotecan to 5FU/LV 6. Capecitabine + oxaliplatin 7. Targeted agents
- 25. • 5-FU–based adjuvant therapy compared to surgery only shows a survival benefit survival for stage III patients.1,2 • Six months of chemotherapy was sufficient, and no further benefit was provided by extending treatment to either nine or twelve months.3,4 • Levamisole, an agent initially thought to be active, was, in fact inactive.5-7 • High-dose LV did not confer superior efficacy over low dose LV and comparisons of various 5-FU/LV schedules did not demonstrate clear superiority of one schedule over the other. • Addition of Oxaliplatin to 5FU provides a significant improvement in DFS and OS especially in Stage III disease8,9. • Capecitabine may be used in lieu of 5FU with comparable outcomes. • Addition of targeted agents (bevacizumab/cetuximab) in the adjuvant setting has not shown any benefit.10,11 Summary of evidence for Adjuvant Chemotherapy in CA Colon
- 26. Adjuvant regimens for Colon cancer Roswell Park (RP) LV: 500 mg/m2, over 2 hrs 5FU: 500 mg/m2 bolus On days 1, 8, 15, 22, 29, 35 Repeat 8 weekly x 32 weeks (4 cycles) Mayo Clinic (MC) LV: 20 mg/m2 d1-d5 5FU: 425 mg/m2 bolus d1-d5 Repeat 4 weekly x 24 wks (6 cycles) Capecitabine X: 850-1250 mg/m2 BiD PO d1-d14 Repeat 3 wkly FLOX LV: 500 mg/m2 over 2 hours weekly 5FU: 500 mg/m2 weekly Ox: 85 mg/m2 before 5FU/LV on d1, 15, 29 Repeat every 8 weeks for 24 wks (3 cycles) FOLFOX4 Ox: 85 mg/m2 on d1 5FU: 400 mg/m2 bolus → 600 mg/m2 continuous infusion (CI) for 22h d1, d2 LV: 200 mg/m2 on d1-2 before 5FU Repeat 2 weekly for 24 weeks (12 cycles) mFOLFOX6 Ox: 85 mg/m2 on d1 LV: 400 mg/m2 on d1 5FU: 400 mg/m2 bolus d1 → 2400 mg/m2 46h CI Repeat 2 weekly for 24 weeks (12 cycles) CapeOx Ox: 130 mg/m2 d1 X: 850-1000 mg/m2 BID PO d1-d14 Repeat 3 wkly for 8 cycles
- 27. • Benefit of adding oxaliplatin to 5FU-LV. • 2,246 patients randomly assigned to receive infusion LV5FU2 (n = 1123) or FOLFOX4 (n = 1123) for 6 months (12 cycles). • Primary end point: DFS. • Secondary end points: - OS - Safety • Median follow-up of 81.9 months. • The planned 12 cycles of CT received by 74.7% and 86.5% of patients in FOLFOX4 and LV5FU2, respectively. Improved Overall Survival With Oxaliplatin, Fluorouracil, and Leucovorin As Adjuvant Treatment in Stage II or III Colon Cancer in the MOSAIC Trial Andre et al, JCO 2009
- 28. CONSORT diagram A:3 pt. assigned to FOLFOX4 arm but did not receive oxaliplatin. Therefore, these patients were considered in FOLFOX4 arm for efficacy analysis and LV5FU2 arm for safety analysis. B:1 pt. assigned to LV5FU2 arm received oxaliplatin. Therefore, this patient was considered in the LV5FU2 arm for efficacy analysis and in FOLFOX4 arm for safety analysis.
- 29. Baseline patient and disease characteristics
- 30. II III FOLFOX (%) 5FULV2 (%) p 5 yr DFS (II + III) 73.3 67.4 0.003 6 yr OS (II + III) 78.5 76.0 0.046 6 yr OS (III) 72.9 68.7 0.023 6 yr OS (II) 86.9 86.8 0.986
- 31. • In stage II disease the difference between the two arms failed to reach statistical significance for both DFS and OS. • In contrast, both differences were significant in case of stage II disease. Conclusion • Adding oxaliplatin to LV5FU2 significantly improved 5-year DFS and 6-year OS in the adjuvant treatment of stage II or III colon cancer.
- 32. • Trial demonstrating OS advantage of XELOX over 5FU/LV. • Randomized 1886 patients to Capecitabine plus oxaliplatin (XELOX, n = 944) vs bolus FU/LV (n = 942, MC or RP) as adjuvant therapy for stage III disease. • Primary end point: DFS • Secondary end points • OS • Relapse-free survival (RFS) • Safety. Capecitabine Plus Oxaliplatin Compared With Fluorouracil/ Folinic Acid As Adjuvant Therapy for Stage III Colon Cancer: Final Results of the NO16968 Trial. Schmoll et al. JCO 2015
- 33. • Treatment-related grade 3/4 adverse events were 55% in XELOX group and 47% in FU/FA group (p < 0.05). • Overall, 69%of pts in XELOX grp and 85% of pts in the FU/FA grp (MC, 87%; RP, 79%) completed the planned number of cycles. • Seven-year DFS rates were 63% and 56% in the XELOX and FU/FA groups respectively (HR 0.80; 95% CI 0.69 to 0.93; p = 0.004) • Seven-year OS rates were 73% and 67% in the XELOX and FU/FA groups respectively (HR 0.83; 95% CI 0.70 to 0.99; P = 0.04) XELOX is better than 5FU/FA as adjuvant chemotherapy in Stage III Colon Cancer.
- 34. Adjuvant therapy in colon cancer: Summary
- 35. • No conclusive evidence of benefit in Stage II without risk factors. • Indicated in stage III, & High risk stage II • FOLFOX or CapeOx are preferred regimens. Addition of oxaliplatin to 5FU increases benefit : - FOLFOX is superior to5FU/LV in terms of DFS & OS. - Benefit for the addition of oxaliplatin to 5-FU/leucovorin in patients ≥ 70 not proven. - Survival benefit not conclusively demonstrated in stage II disease. • Capecitabine/oxaliplatin is superior to bolus 5-FU/ leucovorin. • Capecitabine equally effective to bolus 5-FU/LV.
- 36. Among the various 5FU/LV regimen • Schedule of 5-FU/LV administration does not affect efficacy, but toxicities may be different - Mayo Clinic monthly regimen - more commonly associated with leucopenia and stomatitis - Roswell Park weekly regimen - more commonly associated with diarrhea - Infusional 5-FU/LV may have less toxicity vs. bolus • Irinotecan no benefit in the adjuvant setting. • No targeted agents are approved for use in the adjuvant setting.
- 37. Microsatellite Instability (MSI) • Ribic et al found that adjuvant chemotherapy provide no benefit in patients with a high frequency of MSI (MSI-H). Patients with low frequency of MSI (MSI-L) or no MSI (MSS) continued to derive benefit.1 • However a study by the NSABP2 and a meta-analysis by Webber et al3 failed to show the same finding. • Trials studied by Ribic et al all used 5FU only; positive impact with addition of oxaliplatin is anticipated. • ECOG 5202 results awaited to answer this question. 1. Ribic et al. NEJM 2003 2. Kim et al. JCO 2007 3. Webber et al. BMJ Cancer. 2015
- 38. Adjuvant Radiation therapy in colon cancer Aim • Tumor bed irradiation to decrease local failure. • Conformal delivery to minimize small bowel toxicity
- 39. Rationale • Local failure (LF) in colon cancer depends on stage & location - Anatomic constraints on radial resection margins, - Tumors adherent/ invading adjacent structures increases risk of LF • LF high in ascending/descending colon - Anatomically immobile,” limits wide surgical resection • LF less in mid-sigmoid and mid-transverse colon - Relatively “mobile,” with a wide mesentery, wide margins • LF rates for ceacal, hepatic/splenic flexure, proximal/distal sigmoid tumors are variable - Depending on amount of mesentery present, tumor extension, and adequacy of radial margins.
- 40. Indications Of RT Adjuvant tumor bed irradiation with concurrent 5-FU–based chemotherapy should be considered for patients with tumors • Invading adjoining structures: T4 • Where incomplete resection is performed • Those complicated by perforation or fistula Perez & Brady's Principles and Practice of Radiation Oncology, 6th ed
- 41. Data evaluating the use of adjuvant radiation therapy in high-risk colon cancer patients have largely been limited to single- institution retrospective analyses. • Massachusetts General Hospital (MGH) • Mayo Clinic • University of Florida
- 42. • 222 patients with completely resected : 1) T4 (any site), or 2) T3N+ (of ascending or descending colon only). • PORT 45-50.4 Gy / 25-28# with 5-FU/Levamisole; 5FU for a total 1 year in both arms. • Primary end point: Overall Survival. • Secondary end points - DFS - Patterns of recurrence - Toxicity. • Study was closed early due to poor accrual (Initial goal:700 pts. Total accrual 222 patients between 1992-1996) Adjuvant chemotherapy and radiation therapy compared with chemotherapy alone in the adjuvant treatment of colon cancer: Results of intergroup protocol 0130 Martenson et al. J Clin Oncol 2004
- 44. Results • Median follow-up of living patients was 6.6 years. • No difference in OS or DFS was seen between the two groups.
- 45. • Grade III or IV hematologic toxicity was higher in patients receiving radiation therapy • No significant difference in non hematologic toxicity.
- 46. • Information about pathologic radial margin status was not reliably provided & not used in determination of protocol eligibility. • No single method of determining the tumor volume to guide radiation therapy planning - Clip placement in only 18 (19%) of 94 patients. - Preoperative radiologic imaging (i.e. barium enema or abdominal/pelvic CT): 45 patients (48%). - For 17 patients (18%), the tumor volume defined by operative notes. • 6/94 eligible patients assigned to receive chemo-RT refused RT. Caveats
- 47. Interpretation of study results was handicapped by • Decreased statistical power • High ineligibility rates, • Lack of appropriate target volume definition for Radiotherapy planning No definitive conclusion
- 48. Technique • Bowel preparation • Positioning: Supine, Prone or Lateral decubitus • Immobilization • Contrast: oral & iv - Oral contrast aids in delineating small-bowel, may be useful to compare films in decubitus & supine positions to determine bowel shift • Simulation: Conventional/CT • CT based planning preferred - Facilitates defining tumor bed, beam orientation and estimating OAR doses
- 49. Treatment plans with patient in (A) lateral decubitus and (B) prone position. A B
- 50. Target volume delineation Tumor Bed/Target • Involved segment of colon and, when present, the adjacent structures to which it was adherent or invading • If adherent to partially resected organ: whole organ has to be treated if within tolerance • If adherent to structure (pelvic side wall, psoas, diaphragm): 3-5 cm margins beyond area of adherence • Surgical clips aid in the identification of high-risk areas (i.e., positive margins) to assist in target delineation • The nodal basins in the mesentery beyond surgical margins are usually not treated .However the final inclusion of local & regional nodal group is based on operative & pathologic findings. Margins • Tumor bed covered with a 4- to 5-cm margin proximally and distally and with a 3- to 4-cm margin medially and laterally to cover areas of potential residual disease
- 51. Field Arrangement • Field arrangement will vary, depending on the site of the primary disease, as well as on areas judged to be at high risk for local recurrence • Parallel opposed or other multifield techniques are used to treat target & spare OAR: small bowel, kidney, liver, S.C
- 52. Para-aortic nodes may be at risk, due to tumor adherence to posterior abdominal wall with descending colon cancer. External and common iliac nodes may be at risk, from a proximal ceacal/ascending colon cancer Postop AP-PA Fields Of Extra pelvic Colon Cancer (Tumor Bed And Nodal Regions).
- 53. Dose prescription • Total dose depends on the amount of suspected residual disease and tolerance constraints of surrounding normal tissue. • Initial dose of 45 gy/25 # at 1.8/# delivered through larger fields to primary tumor and at-risk tissues followed by reduced boost fields. • For patients with t4 tumors, the general goal is to treat the tumor bed to a total dose of 50.4-54gy • Any treatment beyond 50 gy generally mandates exclusion of all small bowel from the field
- 54. Critical normal (dose limiting) tissues • Small intestine: 45-50 Gy • Liver: 2/3rd of liver should get <30 Gy • Kidneys: 2/3rd of one kidney should get < 20 Gy • Spinal cord: Max dose to spinal cord < 45 Gy
- 55. Post treatment Surveillance • History, physical examination & S.CEA: - Every 3–6 month for 2 y, then every 6 month for a total of 5 yrs. • Colonoscopy: - In 1st yr: Normal: repeat in 3rd yr then every 5 yrs Abnormal repeat in one yr. If not done previously, 3-6 months post surgery. • CT scan chest & abdomen: - Annually for pts. with high risk for recurrence (eg. LVI/PNI, poorly differentiated tumors). NCCN 2017
- 56. Treatment Options for Metastatic Colon Cancer (Stage IV) with synchronous liver only or lung only metastases 1. For resectable lesions - Colectomy with synchronous resection of liver or lung metastases followed by adjuvant chemotherapy with FOLFOX or CapeOx - Neoadjuvant CT to increase curative resection rates 2. For unresectable lesions - Treated with chemotherapy and evaluated every 2 months to assess resectability of liver and/or lung metastases,colon resection if risk of obstruction or significant bleeding. - Combination chemotherapy for 2-3 months followed by chemo-radiation with 5-FU or capecitabine and then resection of metastases and primary 3. For patients that are able to undergo resection of metastatic disease - 6 months of adjuvant therapy with an active regimen for advanced disease, observation, or shortened course of palliative chemotherapy NCCN 2017, ESMO 2014
- 57. Criteria for resectable metastases • Lung, Liver • Complete resection must be possible while leaving adequate tissue for normal function • Primary disease must have had/be amenable to an R0 resection • If metastatic disease is unresectable, can consider 3-6 months of 5FU based chemotherapy followed by reassessment for resection. • Surgical resection preferred to ablative (eg. SBRT) techniques. • Liver • May consider portal venous embolization or staged resections when non- resectability is based on inadequacy of remnant liver. • Lung • Extra-pulmonary resectable metastases is not a contra-indication. NCCN 2017, ESMO 2014
- 58. Neoadjuvant Chemotherapy i. FOLFIRI, CapeOx, or FOLFOX ± bevacizumab ii. FOLFIRI or FOLFOX + panitumumab if KRAS wild type (WT) iii. FOLFIRI + cetuximab if KRAS WT Adjuvant Chemotherapy Adjuvant First-Line Therapy Adjuvant Second-Line Therapy Good Performance Status • FOLFOX with or without Bevacizumab. • FOLFIRI with or without Bevacizumab. • 5-FU + Leucovrin with bevacizumab If first line Irinotecan • FOLFOX ±Bevacizumab. • Irinotecan ±Cetuximab. • Capecitabine or 5-FU + Leucovorin Poor Performance Status • Capecitabine or 5-FU + Leucovorin ± Bevacizumab. If first line Oxaliplatin • FOLFIRI ± Bevacizumab. • Irinotecan ± Cetuximab. Chemotherapy Options
- 59. Metastatic colon cancer: Chemotherapy • Pts. with unresectable mCRC treated with BSC have poor prognosis (median OS = 5 months). • In contrast, pts. who receive CT have been shown to have a median OS of ≈ 2 yrs.
- 60. Chemotherapy in metastatic colon cancer
- 61. Chemotherapy in metastatic colon cancer
- 62. Targeted agents
- 63. Panitumumab • Indicated in wild type KRAS metastatic colorectal cancer • Approved for use in patients with mCRC refractory to FOLFIRI. • All patients with mCRC should have tumor tissue genotyped for RAS mutations performed in certified laboratories. • The testing can be performed on the primary colorectal cancers and/or the metastasis. Bevacizumab • Monoclonal antibody that binds to vascular endothelial growth factor (VEGF). • Can be added to either FOLFIRI or FOLFOX as first-line & second line treatment of metastatic colorectal cancer.
- 64. Aflibercept • Anti-VEGF molecule evaluated as a component of second-line therapy in patients with metastatic colorectal cancer • FOLFIRI + aflibercept is an acceptable second-line regimen for patients who have failed with FOLFOX-based chemotherapy. Cetuximab • Monoclonal antibody against the epidermal growth factor receptor (EGFR). • Indicated in KRAS wild type, EGFR expressing colon cancer can be added to either FOLFIRI or FOLFOX as first-line treatment of metastatic colorectal cancer. Patients with any known KRAS mutation should not be treated with cetuximab or panitumumab as these render EGFR
- 65. Regorafenib • Inhibitor of multiple tyrosine kinase pathways including VEGF, PDGF, FGFR, B-RAF, RET, KIT • Indicated in mCRC progressed on 5FU,oxaliplatin irinotecan & bevacizumab, aflibercept, cetuximab & panitumumab based chemotherapy. Vemurafenib • Active against the BRAF V600 mutated tumor • BRAF V600 blockade upregulates EGFR. • While not effective as single agent therapy1, in combination with Cetuximab and irinotecan2 benefit in PFS was significant. Absolute benefit, however, was small. 1. Kopetz et al, JCO 2016 2. SWOG 1406, JCO 2017
- 66. Targeted agents summary Drug Dose / Indication Bevacizumab 5-10 mg/kg, 2 weekly with FOLFOX/FOLFIRI Panitumumab 6 mg/kg 2 weekly with FOLFOX Cetuximab 400 mg/m2 loading, f.b. 250 mg/m2 with FOLFIRI Alifbercept 4 mg/kg with FOLFIRI (after progression on oxaliplatin) Regorafenib 160 mg OD 21d (q28 days) After failure on above Vermurafenib* 960 mg BD with cetuximab • Benefit noted only in the metastatic setting. * Not approved.
- 67. Carcinoma of the Rectum
- 68. Strategies Surgery pT1,T2 N0 pT3,T4 or N+ Observation/ adjuvant Adjuvant CMT uT3 or cT4 Preoperative CMT Surgery and post-op chemotherapy
- 69. Surgery • Mainstay of treatment. • Depends on • Stage • Site • After surgical resection alone, local failure is common: • 20% - 50% (average ~ 35%)
- 70. 0: Absence of invasive carcinoma 1: Invasion into head of the polyp. 2: Invasion into neck of the polyp. 3: Invasion into stalk of the polyp. 4: Invasion into base of the polyp. • Polypoid/malignant rectal polyps (T1) based upon the extent of invasion. Haggitt’s classification
- 71. Surgical procedures for Rectal Carcinoma • APR (Abdomino-Perineal Resection, sphincter compromised) • LAR (Low Anterior Resection) • Proctectomy & colo-anal anastomosis Radical • Local excision: - Transanal excision - Post. Proctotomy - Trans-anal endoscopic microsurgery(TEM). Non Radical
- 72. Surgical techniques: Early rectal cancer Local excision • T1N0 or favourable T2N0 lesion <3cm in diameter • <40% circumference of the lumen • <10 cm from dentate line • Well to moderately differentiated histology • No evidence of lymphatic or vascular invasion on biopsy • Mobile, exophitic or polypoid lesions Early rectal cancer: • T1-2N0 (TNM stage I) • Tis and T1 (Japanese classification)
- 73. • Low risk factors - Submucosal invasion <1,000 µm - Haggitt’s level 1-3 • High risk factors: - Heggitt’s level 4 - Poorly differentiated - LVI - Tumor sprouting • Independent poor prognostic risk factors: - Penetration of muscularis propria - Definite vascular invasion - High intensity tumor budding • LN involvement rate - No-risk (0.7%) - One-risk (20.7%) - Multiple-risk factor (36.4%) • By T stage: - T1: 5% - T2: 20% - T3: 65% - T4: 80% Ueno et al. Ann Surg, 2004 Sitzler et al. Dis Colon Rectum, 1997 Low Grade tumor budding High Grade tumor budding
- 75. Local Excision Techniques 1. Transanal Excision. (<3 cm from the dentate line) 2. Posterior proctotomy. (<5 cm from the dentate line) 3. TEM (7-10 cm from the dentate line) • Staging of such lesions should be performed using EUS to minimize likelihood of doing a local excision for T3 tumors. • Advantages: - Rapid recovery, - Minimal effect on sphincter function - Relatively low perioperative morbidity and mortality. • Disadvantages: - Local failure
- 76. Transanal Excision Post proctotomy
- 77. Transanal Endoscopic Microsurgery (TEM) • Special operating proctoscope that distends the rectum with insufflated CO2 and allows the passage of dissecting instruments. • Can be used on lesions located higher in the rectum and even in the distal sigmoid colon. • Significant learning curve and a lack of availability.
- 78. Advanced Rectal Carcinoma: LAR • For tumors in upper/mid rectum allows preservation of anal sphincter • Ocasionally, for lesions in the lower third • No pre-existing sphincter problems • No evidence of extensive local disease in the pelvis. • Join colon to low rectum • Permanent colostomy not required
- 79. • The colon is divided at the sigmoid-descending colon junction • The operation entails full mobilization of the rectum, sigmoid colon, and the splenic flexure. • Mobilization of the rectum is by a technique called total mesorectal excision (TME).
- 80. Total mesorectal excision • Sharp dissection in the avascular plane • Reduces positive radial margin rate. • Specimen gross pathology: - Bilobed - Encapsulated - Smooth & unbroken surface • Local recurrence with conventional surgery averages approx. 25-30% vs. TME 4-7% by several studies. • Disadvantage: anastmotic leak
- 81. Plane of dissection TME Intra-mesorectal excision Muscularis propria
- 82. Radiologic depiction of the TME A. Axial B. Coronal C. Sagittal planes B A C
- 83. Local Recurrence Following Surgery Alone Clinical Colorectal Cancer, 2004;4(4):233-40.
- 84. Total Mesorectal Excision (TME) Clinical Colorectal Cancer, 2004;4(4):233-40.
- 85. Choice of Surgical procedure • Factors influencing sphincter preservation - Surgeon training - Neoadjuvant chemoradiotherapy • Factors associated with difficult sphincter preservation - Bulky tumors within 5 cm from the anal verge - Direct involvement of anal sphincter muscles with carcinoma - Preoperative incontinence - Morbid obesity - Male sex
- 86. Abdominoperineal resection (APR) • For tumors of distal rectum (lower 1/3rd) with distal edge up to 6 cm from anal verge • This procedure involves: - En bloc resection of the tumor, anal sphincters - whole pelvic mesocolon, mesorectum , Wide Perineal dissection - Removal of the lymph nodes - A permanent colostomy
- 87. Outcomes Complications • 5-year survival rates following an APR range from* - 78 to 100% for stage I - 45 to 73% for stage II - 22 to 66% for stage III disease • Urinary complications 50% • Perineal wound infection 16%. • Sexual dysfunction • Stoma complications • Anastomotic leak • Fistula formation * Varying with time and adjuvant treatment APR
- 88. Pelvic Exenteration • The surgeon removes the rectum as well as nearby organs such as the bladder, prostate, or uterus if the cancer has spread to these organs. • A colostomy • Urostomy • Not routinely practiced. • Mohan et al: Systematic review of 22 studies (1,575 patients) - 4.2% perioperative mortality rate with morbidity of 42.5% - The overall 5-year survival rate was 50.3% with worse outcomes in patients with recurrent compared to primary disease (19.5% versus 52.8%). - R0 resection was achieved in 79.5% of cases and, was the strongest factor associated with long-term survival. Mohan et al. Ann Sur Oncol 2013
- 89. Resection Margins • Intramural excision - 2 cm of the distal margin intramural margin - 5 cm of the proximal margin intramural margin • Mesorectal excision - Upper rectal growth – 5cm - Mid and lower rectum – total mesorectum Jemal A,Tiwari RC,murray T, et al . Cancer statistics2004.CA Cancer J Clin2004;54;8-29 Agarwal A,et al. Total mesorectal excision : In:GI Surg Annual ,ed T K Chattopadhyay 2001;(8):57-69. Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–596.
- 90. Circumferential Resection Margin (CRM) • Circumferential radial margin (CRM) is an independent predictor of both local recurrence and survival. Nagtegaal ID, Quirke P. JCO, 2008 Quirke et al. Lancet, 2009 CRM Status LR Rate p value > 2mm Negative 3.3% <0.0001 1to 2mm Close 8.5% 0.02 < 1mm* Positive 13.1% 0.08 * TNM definition of +ve margin is 0mm
- 91. • AJCC/UICC (7th edition) Description Grade No viable cancer cells 0 (Complete response) Single cells or small groups of cancer cells 1 (Moderate response) Residual cancer outgrown by fibrosis 2 (Minimal response) Minimal or no tumor kill; extensive residual cancer 3 (Poor response) Adapted from Ryan et al; Histopathology, 2005 Tumor Regression Grade
- 92. Grade Regression Fibrosis 0 No All cells are viable 1 Minor < 25% fibrosis 2 Moderate 26 – 50% Fibrosis 3 Good >50% 4 Total No Viable Cells Tumor Regression Grade Grade 10 – year DM P 10 – Year DFS P 0 - 1 39.6% 0.005 63% 0.0082 - 3 29.3% 73.6% 4 10.5 % 89.5% Analysis from the German Rectal Cancer Trial. J Clin Oncol 2014;32(15):1554-62.
- 93. • Capirci et al found that in 566 patient who achieved a pCR with neoadjuvant therapy, only 1.6% had a local recurrence and 5 year DFS and OS were 85% and 90% respectively.1 • Others have also reported that patients with a pCR have significantly lower LRR, better DFS and OS compared to those without a pCR2-5. A good pathological response is a good prognostic indicator. Tumor Regression Grade 1. Capirci et al. IJROBP, 2008 2. Kalady et al. Ann Surg 2009 3. Rodel et al. JCO 2005 4. de Campos-Lobato et al, Ann Surg Oncol 2011 5. Maas et al, Lancet Oncol 2010
- 94. Questions • Is adjuvant/neoadjuvant treatment needed? • Preop or Postop? • Concurrent chemotherapy regimen? • Long course or short course? • Ideal gap between RT and surgery? • Role of adjuvant chemotherapy • Role of targeted agents
- 96. Adjuvant treatment • Radiotherapy and/or Chemotherapy • Indications: - T1 lesion after local excision with poor risk (poorly differentiated, margin <3mm, >3cm size and LVSI) - T2 after local excision - All T3-4 or Node positive after surgery
- 97. Drugs used: • 5FU/Leucovorin • Oxaliplatin • Irinotecan • Bevacizumab • Cetuximab Combinations • FOLFOX • Cap-Ox • FOLFIRI • Leucovorin/5FU • Bevacizumab in combination with the above regimens. Adjuvant Chemotherapy
- 99. Adjuvant Therapy Study Description Outcome GITSG 7175 (1988) 4 arm trial S/S+RT/S+CT/S+CRT 227 patients B2 , 10 yr OS 45 % vs 27%,LRR 10% vs 25% Significant benefit with CRT NCCTG 79-47- 51 (1991) Stage II –III RT alone or CRT (5FUX2> 5FU +RT> 5FUX2) (50% APR) 5-yr LR 14% (CRT) vs 25% 5-yr DM 29% vs 46% 5-yr OS 55% vs 45% NSABP R-01 (1988) 3 arm RCT 500 patients (PT3/T4N+) S/S+CT/S+RT CT: Improved DFS & OS RT: Reduction in LRR 16% vs 25 % favouring RT, but no survival benefit. NSABP -02 (1999) 2 arm RCT Postop CT (5FU or MOF) postop CRT PORT: no benefit on DFS or OS, reduced the at 5 yr-LRR (13% to 8%) (P = .02). Post-op CT: 5FU-LV better than MOF: DFS at 5 yr (55% vs 47%; P = .009), 5-year OS (65% vs 62%; P = .17).
- 100. NCI Consensus Statement (1990) • CMT was the standard postoperative adjuvant treatment for all patients with pT3 or N1-2 disease. • Deliver 6 cycle of adjuvant CT (RT given with 3rd & 4th cycle of chemotherapy). • As most US trials used a 5FU continuous infusion based regimen, this is the regimen of choice.
- 101. Neoadjuvant treatment • Preoperative RT alone (Short/Long course) • Pre-operative chemoradiotherapy • Pre-operative chemotherapy
- 102. Preoperative approach: Pros and Cons Pre operative RT Postop RT Pros • Tumour downstaging and better resectability. - Improved chances of sphincter preservation. • Better local control (esp in patients with pCR). Cons • Risk of overtreatment as pathological staging NA. Pros • Therapy is most effective when tumor burden is minimal. • Post op HPE known. Cons • More toxicity - Bowel tethered in pelvis - Perineal scar to be treated • Anastomotic site irradiated, risk of complications.
- 103. Pre-op vs post-op CRT Trial Arms Acute tox Gr 3+ LR OS Sphincter preservation GERMAN cT3-4 cN+ Median FU: 134 mnths Pre op CRT 421 Post op CRT 402 21/27 18/40 7.1% 10.1% p=.048 59.6% 59.9% p=0.85 45/116 37% vs 15/78 19% MRC CR07/NCIC C016 Preop 25 Gy/5# fb Surg Surg + Post-op CRT (if CRM+) - 4% 11% (SS) 4-yr Similar - NSABP R03* Preop (130) Postop CRT (137) 34% 26% 5% 9% 74% 66% 47.8% (55/115)@ 39.2% (47/120) * Recruitment closed after 267/900 (slow accrual) @ Overall numbers; not reported by preop intent • Preoperative CRT is better than post-op CRT in terms of tolerability, local control and to some extent in sphincter preservation. • For sphincter preservation usually follow long course RT schedule.
- 104. • 823 patients randomized to Preop (421) vs Postop (402) CRT • Eligibility criteria • Histopathologically confirmed, resectable adenocarcinoma with the inferior margin within 16 cm from the anal verge. • ERUS and CT scan of the abdomen and pelvis were performed to rule out TNM stage I tumors and distant metastases. • Primary end point: Overall Survival • Secondary end point: • Disease free survival • Local and distant recurrences • Postoperative complications, acute and long-term • Toxic effects • Sphincter preservation Preoperative versus Postoperative Chemoradiotherapy for Rectal Cancer
- 105. Study design
- 107. • Significantly lower incidence of LR; similar OS and Distant failures • Concluded that preoperative CRT improves local control compared to postoperative CRT.
- 108. Preoperative chemoradiotherapy vs RT alone Trial N Randomisation F/U LR (%) OS FFCD 9203 cT3-4 cN+ 733 Pre op CRT f/b Sx Preop RT f/b Sx - 5yr 8.1% Vs 16.5% P = 0.048 5yr NSS EORTC 22921 4 arm study 1011 Pre op RT → Sx → + CT Preop CRT → Sx → + CT 10.4 Yrs (7.8- 13.1) 18.4 11.8 p = 0.0017 (at 10 years) 22.4 & 14.5 11.8 & 11.7 49.4 50.7 p = 0.91 (at 10 years)
- 109. So far.. • Surgery (TME) should be followed by (when indicated) adjuvant chemoradiotherapy. • However, preoperative RT offers better local control than postop RT though there is no demonstrable survival benefit. • Concurrent chemotherapy should be given along with RT.
- 110. Optimizing Concurrent chemotherapy Trial Chemo Sample RT pCR (%) Ac. Tox. 3+ (%) STAR-01 5FU 5FU+Ox 379 368 50.4/28 16 15 8 24 ACCORD 12/ 0405 PRODIGIE Cap Cap+Ox 295 291 45/25 50.4/28 14 19 11 23 NSABP R-04 (3 yr) 5FU+Ox Cap+Ox 806 802 50.4/28 54/30 (T4) 17.8 20.7 28.2 & 40.1 (Ox) 32.9 & 41.9 (Ox) CAO/ARO/ AIO-94 (50 mnth) 5FU 5FU+Ox 637 628 50.4/28 13 17 23 22 • Large randomized trials have failed to show any difference between capecitabine & 5FU or benefit with adding oxaliplatin. 5 Fluorouracil (CI) or capecitabine are the agents of choice in the concurrent setting.
- 111. Relative advantages of Neoadjuvant RT Short-course Long Course 1. Shorter duration 2. No chemotherapy 3. Patient taken up for definitive management earlier. 4. Less acute toxicity → Better patient compliance 1. Downstaging of disease 2. Better chances of pCR 3. Time for recovery from acute toxicity 4. Sphincter preservation may be possible.
- 112. Short Course Preoperative Radiotherapy Study Outcome Swedish Rectal cancer Trial PreopRT vs Surg alone, cT1-3 • N = 1168 • 25Gy/5Fr/5days → Surg • Med FU 13 years • OS 38% vs 30% p 0.008 • LRR 9%vs 26% p 0.008 Dutch study CKVO 95-04 PreopRT vs TME alone, cT1-3 • N = 1861 patients • 25Gy/5Fr/5days→TME • Median FU 12 years OS 48%vs 49% p 0.86 LR 5% vs 11% p 0.0001 • RT alone; concurrent chemotherapy not given as ↑ toxicity anticipated. • Swedish trial found an OS benefit while the Dutch trial didn’t; possibly due to standardization of surgery. - Generally higher OS and DFS in the TME study.
- 113. Short Course RT vs Long Course Chemoradiotherapy Study No of Patients Randomis ation Median F/U 3 yr LRR 5 yr OS Toxicity late Australian Intergroup trial 2012 326 T3 N0-2 M0 SC – 163 LC – 163 5.9yrs 7.5% Vs 4.4% P – 0.24 74% Vs 70% P – 0.62 G 3-4 5.8 vs 8.2 P-0.53 Polish rectal cancer group 2006 312 SC:156 LC 156 48 mths Higher pCR in CRT 67.2% Vs 66.2% 10.1% Vs 7.1% • Tumour downstaging/higher pCR with CRT. • No conclusive evidence of survival benefit/sphincter sparing; both small trials.
- 114. Gap between NA-RT and Surgery Neoadjuvant Chemoradiation Lyon R90-01 1999 RCT < 2 wks: 99 6-8 wks: 102 39 Gy / 13# 6-8 weeks DS+ SS- Moore 2004 RR < 44 days: 82 > 44 days: 73 48.6 – 50.4 Gy / 26 – 28# > 44 days pCR+ SS- Kalady 2009 RR 242 50.4 Gy / 28# (median) > 8 weeks pCR+ LR+ Sloothaak 2013 RR 1593 50-50.4 Gy / 25 - 28# > 16 wks (for a maximal CR) pCR+ RCT: Randomized Control Trial RR: Retrospective review PS: Prospective series pCR: pathological Complete response SS: Sphincter preservation DS: Downstaging
- 115. Short Course RT Stockholm III 2017 RCT SRT < 7 days: 228 SRT 4-8 wks: 227 CRT 4-8wks SRT: 25 Gy / 5# LRT: 50 Gy / 25# 4-8 wks pCR+ TRG+ CRM- Pach et al 2012 RCT SRT < 10 days: 77 SRT > 4 wks: 77 25 Gy / 5# > 4 weeks; OS benefit in DS+ DS+ SS- Graf et al 1997 1316 25-25.5 Gy / 5-7# > 10 days DS+ Conclusion: • 4-8 weeks gap is the present standard of care after CTRT, while < 7 days is the norm after short course RT. • There is a case for increased interval after short course RT. More evidence awaited. Gap between NA-SRT and Surgery
- 116. Optimal fractionation of preoperative radiotherapy and timing to surgery in rectal cancer: Stockholm III • 3 types of treatment • Short Course RT → Surgery (SRT) • SRT → Delay (4-8 weeks) → Surgery (SRT delay) • Long Course RT (LRT) → Delay (4-8 weeks) → Surgery (LRT-delay) • Primary end point: Time to local recurrence • Secondary end points: • Overall survival • Frequency of postop mortality • Frequency of postoperative complications • Frequency of reoperations • Frequency of late complications • Radiation toxicity • Frequency of Sphincter-preserving surgeries Erlandsson et al. Lancet Oncol, 2017 • Resectable adenocarcinoma • < 15cms from anal verge • Total: 840 patients • Median FU: 5.2 years
- 117. Stockholm III: Trial design Erlandsson et al. Lancet Oncol, 2017
- 118. • In patients with any LR, median time from randomisation to LR was 33.4 months (range 18.2-62.2) in the SRT group vs 19.3 months (8.5-39.5) in the SRT-delay group. Median time to LR in the CRT group was 33·3 months (range 17·8-114·3). • Cumulative incidence of LR in the whole trial was 8 of 357 in SRT, 10 of 355 in SRT-delay, and 7 of 128 in CRT (p = 0.48) • SRT-delay vs SRT: HR 1·44 [95% CI 0·41-5·11] • SRT-delay vs CRT: HR 2·24 [95% CI 0·71-7·10] • Acute RT toxicity was recorded in one patient (<1%) of 357 after SRT, 23 (7%) of 355 SRT-delay, and six (5%) of 128 after CRT. • Frequency of postoperative complications was similar between all arms when the three-arm randomisation was analysed, but in a pooled analysis of the two SRT regimens the risk of postop complications was significantly lower after SRT-delay than SRT (OR 0·61 [95% CI 0·45-0·83] p=0·001). Erlandsson et al. Lancet Oncol, 2017
- 119. Erlandsson et al. Lancet Oncol, 2017
- 120. Erlandsson et al. Lancet Oncol, 2017
- 121. Local recurrence (A), distant metastases (B), recurrence-free survival (C), and overall survival (D) in the three-arm randomisation with a minimum of 2 years of follow-up Erlandsson et al. Lancet Oncol, 2017
- 122. Local recurrence (A), distant metastases (B), recurrence-free survival (C), and overall survival (D) in the pooled short-course radiotherapy comparison with a minimum of 2 years of follow-up. Erlandsson et al. Lancet Oncol, 2017
- 123. Conclusion • Delaying surgery after short-course radiotherapy gives similar oncological results compared with short-course radiotherapy with immediate surgery. • Quality of life outcomes and sphincter preservation rates yet to be reported.
- 124. Neoadjuvant Therapy: Adding EGFR Inhibition Rodel et al. Curr Opin Oncol, 2012
- 125. Neoadjuvant Therapy: adding VEGF Inhibition Rodel et al. Curr Opin Oncol, 2012
- 126. Neoadjuvant therapy: Targeted agents • As of now, not recommended outside a clinical trial. (EGFR / VEGF inhibitors) • Use in the adjuvant setting under evaluation.
- 127. Is adjuvant Chemotherapy needed for all patients? • Adjuvant 5FU was associated with an improvement in overall survival (OS) in patients with advanced disease from the era preceding TME. • Changes 1. Adoption of TME 2. Increasing use of neoadjuvant radiation 3. Improved staging accuracy 4. Addition of Oxaliplatin to standard regimens Fisher et al. JNCI 1988 Petersen et al. Cochrane Database Syst Rev 2012
- 128. Modern trials evaluating adjuvant chemotherapy Trial Chemo Regimen Survival data DFS OS C O p C O p EORTC 229211 5FU/LV 10 years 47 44 .29 52 48 .32 CHRONICLE2 Cap-Ox 3 years 77 71 .56 88 89 .55 PROCTOR/ SCRIPT*3 5FU/LV or Cap 5 years 63 55 .13 80 79 .73 Italian4 5FU/LV 5 years - - - 68 70 - ADORE5 5FU/LV vs FOLFOX 3 years 72 63 .047 - - - C: Chemotherapy (adjuvant); O: Observation; p: p value * Recruitment closed after 437/470 (slow accrual) 1. Bosset et al. Lancet Oncol, 2014 2. Glynne-Jones et al. Ann Oncol 2014 3. Breugom et al. Ann Oncol, 2015 4. Cionni et al. Radiother Oncol 2010 5. Hong et al. Lancet Oncol, 2014
- 129. Adjuvant chemotherapy: Conclusion • For patients who have received neoadjuvant CRT, adjuvant chemotherapy (6 months of FOLFOX or XELOX) is recommended for Stage II disease and beyond. • In an era with improved CMT, benefit needs to be re- evaluated. • Observation may be a reasonable approach for a subset of patients with early disease (yp Stage II, node negative), further follow up and outcome results needed.
- 130. Questions Additional treatment needed? Yes Preop or Postop? Preoperative Concurrent chemotherapy regimen? 5FU or Cap Long course or short course? ? Individualize Ideal gap between RT and surgery? CRT: 6-8 weeks SRT: < 7 days; ? > 4 weeks Role of adjuvant chemotherapy Recommended*, may change with further evidence. Role of targeted agents Not recommended* *NCCN 2017; FOLFOX/Cap-Ox
- 131. Unresectable Locally advanced rectal cancer • T4 tumors with deep local invasion into adjacent structures, which makes primary resection for cure difficult, invade into pelvic sidewall or sacrum. • Pre-operative chemoradiation • IORT • Pre-operative chemotherapy
- 132. Future directions • Chemotherapy given before Surgery? - PROSPECT: Recruiting T2N1, T3N0-1 and randomizing to NACT + CTRT → Surg - RAPIDO: Sequential SRT → NACT → Surgery. • Delay after short course RT? - Stockholm III suggests SRT-delay is a feasible option. Long term outcomes and sphincter preservation rates awaited. • Chemotherapy concurrent with Short course RT?
- 133. Strategy Surgery pT1,T2 N0 pT3,T4 or N+ Observation/a djuvant Adjuvant CMT uT3 or cT4 Preoperative CMT Surgery and post-op chemotherapy
- 134. RT Techniques
- 135. Positioning • Supine or Prone position with belly board • Prone position displaces bowel loops. • Bladder distension • Marker at anal verge • Small bowel contrast
- 136. Pelvic subsites Five subsites: predominant areas at risk for local recurrence: • Mesorectal subsite (MS): less common as it gets totally removed in TME • Posterior pelvic subsite (PPS): most common site of nodal recurrence 22 to 49% in various series. • Lateral pelvic subsite (LPS): 21% • Inferior pelvic subsite (IPS) : 3-11% • Anterior pelvic subsite (APS): 5-17%
- 137. Field design • PA portal with 6MV to decrease bladder dose • Lateral portals may require higher energy (10/15 MV) to increase depth dose and decrease dose to hip joints • Portals • 4 fields (AP, PA, two lateral fields) • 3 fields (PA, two lateral fields) • 2 fields (AP-PA) • Typically a two phase treatment • Phase I: Whole pelvis including nodes. • Phase II: Boost to tumor (or tumor bed).
- 138. Field Arrangement: Whole pelvic field AP-PA • Lateral borders: 1.5 cm lateral to the widest the true pelvic • Inferior border: 3 cm below the primary tumor or at the inferior aspect of the obturator foramina, whichever is the most inferior • Superior border: L5-S1 junction. • For APR patients, inferior border to be shifted to include the post-op scar.
- 139. Laterals • Posterior border: 1 to 1.5 cm behind the anterior bony sacral margin. • Anterior border: Posterior or anterior margin of the symphysis pubis • Placing it anteriorly includes the external iliac nodes within the field. Field Arrangement: Whole pelvic field
- 140. • Intent: Treat the primary tumor bed plus a 3-cm margin. • After an abdominoperineal resection: • 1.5-cm margin beyond the post-op scar to be treated. • Therefore, scar to be marked with radio-opaque wire during simulation. • Blocks are used to spare the posterior muscle and soft tissues behind the sacrum and small bowel. Field Arrangement: Boost field
- 141. Fig B: For a T4N1M0 rectal cancer 8 cm from the anal verge. Since the tumor was a T4, the anterior field is at the anterior margin of the symphysis pubis (to include the external iliac nodes). Fig A: Treatment fields after a low anterior resection for a T3N1M0 rectal cancer 8 cm from the anal verge. The distal border is at the bottom of the obturator foramen and the perineum is blocked. Since the tumor was a T3, the anterior field is at the posterior margin of the symphysis pubis (to treat only the internal iliac nodes). Fig C: Treatment fields following an abdominoperineal resection for a T4N1M0 rectal cancer 2 cm from the anal verge, because the tumor was a T4, the anterior field is at the anterior margin of the symphysis pubis (to include the external iliac nodes). Since the distal border is being extended only to include the scar and external iliac nodes, the remaining normal tissues can be blocked
- 142. Dose • Preoperative radiotherapy • Short course: 25 Gy in 5 daily fractions of 5 Gy given in 5 days. • Long course Phase 1 45 Gy in 25 daily fractions of 1.8 Gy given in 5 weeks. Phase 2 5.4–9 Gy in 3–5 daily fractions of 1.8 Gy • Postoperative radiotherapy Phase 1 45 Gy in 25 daily fractions of 1.8 Gy given in 5 weeks. Phase 2 5.4–9 Gy in 3–5 daily fractions of 1.8 Gy.
- 143. Rectal Cancer Contouring Guidelines • New definitions for subsites: • PS: Pre-Sacral Nodes - Abdominal - Pelvic • M: Mesorectum • LLN: Lateral Lymph Node - Anterior - Posterior • EIN: External Iliac Nodes • IN: Inguinal Nodes • IRF: Ischio-rectal fossa • SC: Sphincter Complex Valentini et al, Radiother Oncol 2016
- 144. • Mesorectum (M) • Cranial: Bifurcation of the IMA in SA and SRA • Caudal: Insertion of the levator ani muscle into the external sphincter muscles (disappearing of the mesorectal fat around the rectum) • Anterior: • Superior: 7 mm beyond SRA excluding bowel structures • Mid/inferior: mesorectal fascia, posterior border of the anterior pelvic organs • Posterior: Anterior surface of the sacrum and coccyx to the level of IRF (including the medial part of the PS) • Lateral: • Upper/mid: Mesorectal fascia if visible or medial border of the LLN and EIN • Lower: medial edge levator ani muscle
- 145. Pre-sacral nodes (PS) are divided into an abdominal and a pelvic part, because the abdominal part doesn’t have to be treated in all patients. • Limits: • Abdominal - Cranial: Aortic bifurcation/5mm above cranialmost positive node - Caudal: Sacral promontory - Anterior: 1 cm ventral to lumbar vertebrae - Posterior: Lumbar vertebrae anterior wall • Pelvic - Cranial: Bifurcation of the common iliac arteries/promontory - Caudal: Caudal border of Mesorectum (M) - Anterior: 1 cm ventral to lumbar vertebrae - Posterior: Lumbar vertebrae anterior wall
- 146. • Lateral Lymph Nodes (LLN) correlate with a triangular area between the pelvic wall and mesorectum.` • Divided into an anterior and a posterior part; this division is at a virtual coronal plane crossing the anterior wall of the ureters when they join the bladder and the posterior aspect of the external iliac vessels cranially. • Posterior group corresponds to the internal iliac nodes. • It is suggested that for early disease (pT3N0 with MRF-) the cranial limit of the internal iliac nodes (i.e. Posterior LLN) may be lowered at the level of the bifurcation of IMA. Lateral Lymph Nodes
- 147. • Cranial: Bifurcation of common iliac artery • Caudal: Insertion of the levator ani muscle into the external sphincter (pelvic floor) • Anterior • Upper pelvis: 7 mm around the vessel. • Mid pelvis: a virtual coronal plane crossing the anterior wall of the ureters when they join the bladder and the posterior aspect of the external iliac vessels cranially • Inferior pelvis: posterior limit of the obturator fossa • Posterior: Lateral edge of the sacro-iliac joint • Medial: • Upper: Above the M add 7 mm around the vessel, excluding normal anatomic structures • Mid/lower: Mesorectal fascia, pelvic organs • Lateral • Upper: iliopsoas, pelvic bones • Mid-lower: medial edge of the pelvic wall muscles (pyriform and internal obturator muscles) Borders for the posterior LLN
- 148. • The posterior border is the same as the anterior border of the posterior LLN: • Upper pelvis: 7 mm around the vessel. • Mid pelvis: a virtual coronal plane crossing the anterior wall of the ureters when they join the bladder and the posterior aspect of the external iliac vessels cranially • Anterior • Mid pelvis: posterior wall of the EIN • Low pelvis (when external iliac vessels leave the pelvis): anterior surface of obturator artery • The cranial, caudal and lateral borders remain the same. Borders for the anterior LLN Anterior border of the posterior lateral node when the ureters join the bladder Include when: • Positive nodes in the posterior LLN • cT4 • Multiple positive nodes (N2)
- 149. External Iliac Nodes (EIN) • Located around the external iliac vessels • Borders: • Cranial: bifurcation of common iliac artery into internal and external iliac arteries • Caudal: Where the deep circumflex vein crosses the external iliac artery. Alternatively between the acetabulum roof and the superior pubic rami • Anterior: 0.7 cm anterior to the vessels. 1.5 cm antero-laterally along the iliopsoas muscle to include the antero-lateral nodes • Posterior: posterior border of the external iliac vein • Medial: 7 mm medial to the vessel, excluding pelvic organs • Lateral: the iliopsoas muscle
- 150. • To be included when: - cT4 - positive anterior LLN Caudal border of the external iliac nodes (orange), where the deep circumflex vein crosses the external iliac artery External Iliac Nodes (EIN)
- 151. Inguinal Nodes (IN) • Cranial: Where the deep circumflex vein crosses the external iliac artery. Alternatively (if difficult detection on CT images) between the acetabulum roof and the superior pubic rami (i.e. continues below where the EIN end) • Caudal: Where the great saphenous vein enters the femoral vein • Anterior: at least 20 mm margin around inguinal vessels including any visible lymph nodes or lymphoceles • Posterior: the femoral triangle formed by iliopsoas, pectineus and abductor longus muscles • Medial: 10-20 mm margin around the femoral vessels including any visible lymph nodes or lymphoceles • Lateral: medial edge of the sartorius or iliopsoas muscles To be included in • Positive IN • Anal canal/external anal sphincter infiltration • cT4 with infiltration of the lower third vagina
- 152. Ischiorectal Fossa (IRF) • IRF is the fatty triangular area framed by the levator ani muscles, the obturator and gluteus muscles and the ischial tuberosity. Borders • Cranial: Where the inferior pudendal artery leaves the pelvis • Caudal: Oblique plane joining the inferior level of SC and the ischial tuberosity. • Posterior: • Mid-superior: Major gluteus muscle • Inferior: Virtual line tangent to the posterior level of the sphincter • Medial: Levator ani muscles • Lateral: Ischial tuberosity, internal obturator muscle, Gluteus maximus muscle
- 153. • Included when there is infiltration of the external anal sphincter or the ischio-rectal fossa. Cranial border of the ischio-rectal fossa (blue), where the inferior pudendal artery leaves the pelvis Caudal border of the ischio-rectal fossa (blue), at the inferior level of the sphincter complex and the ischial tuberosity Ischiorectal Fossae
- 154. CTV • Always treated: M, pelvic PS and the posterior LLN • Optional: anterior LLN, the SC, the IRF and the IN depends on tumor stage and tumor location.
- 155. Organs at risk (OAR) : Dose constraints Small bowel Bladder Femoral head V180cc < 35 Gy V100cc < 40 Gy V65cc < 45 Gy V40 < 40 Gy V15 < 45 Gy V40 < 40 Gy V25 < 45 Gy
- 156. Intra-Operative Radiotherapy (IORT) • IORT delivers a concentrated dose of radiation therapy to a tumor bed during surgery • Dose range from 1250-2000cGy. • Reserved for pts. with early-stage disease. • Delivered immediately after tumor is removed, helping to destroy the microscopic tumor cells that may be left behind • Spares healthy tissues and organs • Shortened treatment times.
- 158. Endocavitary Radiation • Alternative to local excision/conservative treatment • Selection criteria: - Small T1 - Mobile T2 lesion - Less than 10 cm from the anal verge - No larger than 3 cm. • A total of 6 application of high-dose (20Gy to 30 Gy), low- voltage radiation (50kV) is given over the course of 6 weeks. • Dose at 1 cm depth = 1/3 rd of surface dose
- 159. Treatment cones for Endocavitary radiotherapy
- 160. • Cumulative surface dose might be high but well tolerated. • The overall survival rate is 83%, although the local recurrence rate as high as 30% • In combination with EBRT may be considered in medically unfit patient for surgery. • Drawbacks : • Not suitable for tumors extending into anal canal • May develop transitory superficial ulcer
- 161. Carcinoma Anal Canal
- 162. Staging T Stage Tx Primary tumour cant be assessed T0 No evidence of primary tumour Tis Carcinoma in situ (Bowen's disease, high-grade squamous and anal intraepithelial lesions) T1 Tumor 2 cm or less in greatest dimension T2 Tumor more than 2 cm but not more than 5 cm in greatest dimension T3 Tumor more than 5 cm in greatest dimension T4 Tumor of any size that invades adjacent organ(s); e.g., vagina, urethra, bladder AJCC/UICC 7th edition Carcinoma in situ (Bowen's disease, high-grade squamous and anal intraepithelial lesions) HSIL AJCC/UICC 8th edition
- 163. T1 T3 T2 T4 2-5 cm >5 cm T Staging
- 164. Staging N Stage Nx Regional lymph nodes cant be assessed N0 No regional nodal metastases N1 Metastasis in perirectal lymph node(s) N2 Metastasis in unilateral internal iliac and/or inguinal lymph node(s) N3 Metastasis in perirectal and inguinal lymph nodes and/or bilateral internal iliac and/or inguinal lymph nodes AJCC/UICC 7th edition
- 166. N1 N2 N2 N3 N3 N3
- 168. Staging N Stage Nx Regional lymph nodes cant be assessed N0 No regional nodal metastases N1 Regional lymph node(s) metastases N1a Metastasis in mesorectal, inguinal or internal iliac lymph node(s) N1b Metastasis in external iliac lymph nodes N1c External iliac lymph nodes with any N1a nodes AJCC/UICC 8th edition
- 170. Overall Stage AJCC/UICC 8th edition
- 171. Management Algorithm Gunderson 4th edition
- 172. Surgery: APR • Standard of care for the primary treatment of anal carcinoma before the adoption of CRT. • Average 5-year OS with APR alone reportedly were 50% (range, 25%-75%). • LRR and distant failure occurred in up to 35% and 10% patients, with higher rates for positive pelvic or inguinal lymph nodes. • High morbidity and rates of recurrence; now reserved for salvage.
- 173. • Till the late 1970s, the treatment for anal cancer was an APR. • Nigro et al1 first reported on 3 consecutive patients planned with Chemo-RT followed by APR, who all achieved a pCR. This was updated for 28 patients2, with 23 attaining a pCR. • With a FU of 1-9 years, 22/28 patients were alive and 19 were disease free (12 APR, 14 excisions, all 6 deaths in APR patients). The Nigro Regimen 1. Nigro et al, Dis Colon Rectum 1974 2. Nigro et al, Cancer 1983 External Irradiation: • 3000 rads to anorectal and local nodal areas on d1 - 21. • 200 rads/day/15 days Systemic Chemotherapy A. 5-FU: I000 mg/m2;24 hr as a continuous infusion for 4 days. Start on day 1. B. Mitomycin C: 15 mg/m2 bolus on day 1. C. 5-FU: Repeated day 20 to 31.
- 174. Chemo-RT vs. RT Alone • UKCCCR ACT I • EORTC
- 175. UKCCCR Anal Cancer Trial (ACT) 1 • 577 patients randomized to RT alone (285) or CRT (292). • 45 Gy phase I → 6 week break → 15 Gy boost OR Salvage APR • MMC 12 mg/m2 d1; 5FU 1g/m2 d1-4 and d29-32 • Primary end point: Local Failure • Secondary end point: 5 year survival • Initially calculated sample size was 130 per arm to show a 60% reduction in local failure; 585 patients were recruited. • Median FU: 13 years UKCCCR. Lancet 1996; upd. Northover et al, Br J Cancer 2010
- 176. • Updated results after 13 years of follow-up showed a non significant survival advantage for the chemoradiation arm, but cancer specific survival was significantly improved. • In patients who underwent an APR, 5 yr survival was > 50% for node negative patients vs 20% for N+. CR LC DM OS CRT 39 61 10 65 RT Alone 30 39 17 58 P 0.08 < 0.001 NR 0.25 * All values in percentages UKCCCR. Lancet 1996; upd. Northover et al, Br J Cancer 2010 Conclusion • Chemoradiation was superior to RT alone for almost all evaluated end points, and should be the standard of care.
- 177. CT-RT vs RT Alone: a Phase III EORTC trial • 110 patients T3-4N0-3 or T1-2N1-3 (locally advanced ds) • CRT: 52 patients (45 Gy → 6 week break → 15–20 Gy boost, concurrent 5FU 750mg/m2 d1-4, d29-32 + mitomycin C 15 mg/m2 d1) vs RT alone: 51 patients. • End points - Overall Survival - Colostomy free survival - Local Control - Event Free Survival - Severe toxicity free interval • Salvage surgery for < 50% reduction; patients achieving a CR with salvage surgery were deemed treatment successes. Bartelink et al. JCO 1997
- 178. • 3 factors were significantly associated with poorer survival - Skin ulceration, nodal involvement and male sex. (Prognosis did not differ by N1 vs N2 stage). Conclusion: • The combination of RT and CT resulted in an improvement in local control and a reduction in the need for colostomy in patients with locally advanced anal cancer. CR* 5 yr LC CFS PFS OS ChemoRT 80 68 72 61 57 RT Alone 54 50 40 43 52 p NR 0.02 0.002 0.05 0.17 All values in percentages; * CR: Complete remission. Bartelink et al. JCO 1997
- 179. Concurrent Chemotherapy Regimens Is MMC necessary: RTOG 87-04/ ECOG 1289 CDDP or MMC: ACT II, RTOG 98-11
- 180. Role of MMC: RT + 5FU + MMC RTOG 8704 / ECOG 1289 • 291 patients (any T, any N), 146 MMC arm and 145 to test arm. • End points - Negative postinduction biopsy - Incidence of Positive salvage biopsy - Local regional control - Time to colostomy - Colostomy-free survival - Disease-free survival - Overall survival - Toxicity rate • Patients with residual disease post RT were considered failures. • Median follow up: 3 years Flam et al, JCO 1996
- 182. A. Two-field (AP-PA) technique. Superior border lowered to bottom of SIJ at 30.6 Gy and lower corner blocks were not used. At 36.0 Gy, 10- x 10-cm fields with unchanged inferior borders were used. B. When inguinal nodes were involved, anterior field flared to include both inguinal regions. Shaded area was boosted by electrons or photons to bring nodal dose at 3-cm depth to 50.4 Gy.
- 183. -ve Biopsy +ve Biopsy Colostomy CFS PFS OS 5FU + MMC 92 8 9 71 73 78 5FU 86 14 23 59 51 71 p 0.135 0.135 0.002 0.014 0.0003 0.32 • Patients with primary tumors less than 5 cm achieved a 93% -ve biopsy rate vs with 83% for patients with bigger primary tumors (p = .02). • The impact of MMC on colostomy rate reduction was most significant in T3/T4 primary tumors (p = .019) vs T1/T2 primary tumors (p = .141). Conclusions: • Adding MMC to 5-FU and RT improves postinduction biopsy rates, colostomy rates, CFS and DFS. Flam et al, JCO 1996
- 184. 2x2 trial Cisplatin vs MMC: the ACT II trial • Primary end point: Complete response (at 26 weeks) • Secondary end points - colostomy-free survival - in-field recurrence rate - cause-specific survival - Overall survival - Progression-free survival Median follow up 5.1 years
- 185. RT: Phase I: 30.6 Gy / 17# Phase II: 19.8 Gy / 15# 5FU: 1000 mg/m2/d, d1–4 & 29–32. Mitomycin C: 12mg/m2, d1 (max 20mg) Cisplatin: 60mg/m2, d1 & d29 (max 120mg) Adjuvant: CDDP 60mg/m2, 5FU 1000mg/m2 • Stage T1–T2 (50%), T3–T4 (43%), LN-(62%), LN+ (30%) • Stratified by site, T and N stage, sex, age & renal function • 90.5% patients in the MMC arm vs 89.6% in the CDDP arm had a CR at 26 weeks (p = 0.64). • 3 year PFS was 74% (CDDP) vs 73% (MMC); p = 0.63). • 3-year CFS was 73%, 75%, 75% and 72% in the MMC /maintenance, CDDP/maintenance, MMC/No maintenance and CDDP/No maintenance groups respectively.
- 186. • Overall, toxic effects were similar in each group [71%] mitomycin vs [72%] cisplatin). • MMC patients had more acute grade 3/4 hematological toxicities (25 vs. 13%). Conclusion: 5-FU + MMC with RT remains the standard of care. PFS OS
- 187. • Patients with T2-T4, any N anal carcinoma (65% T2, 70% N0, 48% Stage I) • Stratifications: Sex, nodal status and tumor diameter. Cisplatin vs MMC: RTOG 98-11 Ajani et al • RT: 45 Gy (30.6 Gy Phase I, 14.4 Gy Phase II) • 5FU 1000 mg/m2 on d1-4, d29-32 MMC arm; additionally on d57-60 and d85-88 CDDP arm • CDDP 75 mg/m2 on days 1, 29, 57, 85 • MMC 10 mg/m2 on days 1 and 29 NACT (2 x CDDP/5FU) fb Chemoradiotherapy • Concurrent 5FU/CDDP CR Follow up T3. T4, N+ or residual T2 Boost 10-14 Gy Chemoradiotherapy • Concurrent 5FU/MMC versus 320 324
- 188. Ajani et al. JAMA 2008 AP-PA fields Four field
- 189. • Primary end point: 5-year disease-free survival • Secondary end points: • overall survival • Time to relapse DFS OS Colostomy LRR DM Hemat toxicity MMC 60 75 10 25 15 61 CDDP 54 70 19 33 19 42 p 0.17 0.10 0.02 0.07 0.17 < 0.001 NR: Not reported; all values in percentages Ajani et al. JAMA 2008 • Severe acute hematologic toxicity was worse in the MMC arm, but all other toxicities were comparable. Long term toxicity was also similar.
- 190. RTOG 98-11: Long term update Gunderson et al. JCO 2012 • DFS and OS were statistically better for MMC versus CDDP (5-year DFS, 67.8%v 57.8%; P = .006; 5-year OS, 78.3% v 70.7%; P = .026). • CFS (P = .05), LRF (P = .087) and CF (P = .074) were numerically better. Conclusion • CCR with FU/MMC remains the standard of care for Anal SCC.
- 191. Other approaches • Radiotherapy intensification: ACCORD 03 • Neoadjuvant chemotherapy: CALGB 9281, ACCORD 03 • Targeted agents
- 192. Induction Chemotherapy and RT Dose Intensification: the UNICANCER ACCORD 03 study Peiffert et al. JCO, 2012 2x2 factorial design ICT: Induction Chemo; RCT: Chemoradiotherapy; SD: Standard dose; HD: High dose • ICT: 5FU 800 mg/m2/d CI, d1-4 & 29-32; CDDP 80 mg/m2 d1 & d29 • RCT: 45 Gy / 25# / 5 wks; 5FU * CDDP during weeks 1 and 5 • HD: 20-25 Gy • SD: 15 Gy • 307 Patients with Tumors ≥ 40 mm or node positive Median FU: 50 months
- 193. Conclusion • Using CFS as primary end point, there was no advantage for either ICT or HD radiation boost in LAACC. Peiffert et al. JCO, 2012 CFS OS LC Induction vs No Induction 76.5 vs 75 P = 0.37 74.5 vs 71 P = 0.81 80.3 vs 81 P = 0.65 Standard Dose vs High Dose 73.7 vs 77.8 P = 0.067 71 vs 74 P = 0.43 78.2 vs 83.1 P = 0.28 • Primary end point: Colostomy Free Survival • Secondary end points - Overall survival (OS) - Local control (LC) - Cancer-specific survival
- 194. CALGB 9281 • Phase II - 45 patients. • NACT (T3/T4 and/or N2/N3) . • 2 week break included. • Four-year data: OS 68%, DFS 61, 23% colostomy rate. • No evidence to suggest improvement - May be used in cases of abscess or fistula. Meropol et al, JCO 2008 2 x NACT: CDDP/5-FU EBRT 45 Gy 2 x Concurrent MMC/5-FU Single cycle CDDP/5FU + 9 Gy boost
- 195. Role of Cetuximab • 23 patients in a phase 2 study of chemoradiation with 5FU, cisplatin, and cetuximab.1 • RT: 55 to 59 Gy over 6.0 to 6.5 weeks. • Response rate of 95%, but closed early: - 6 (26%) episodes of thrombosis/embolism - 52% grade 3 or 4 radiation dermatitis - 44% grade 3 or 4 diarrhea. • ACCORD 16 phase 2 trial evaluating cetuximab concurrently with chemoradiation for anal cancer was closed early because of excessive toxicity. • At present, no role in definitive setting. May be used in metastatic disease in combination with cytotoxic agents.2 1. Olivatto LO et al Cancer. 2013 2. Lukan et al. Oncology 2009
- 196. Impact of OTT on outcomes: Pooled analysis of RTOG 8704 and 9811 • Univariate analysis • Significant association between Rx duration and • Colostomy failure HR 1.51 • Local failure HR 1.52 • Locoregional failure HR 1.51 • Time to failure HR 1.40 Patients with a median Rx time more than 53 days had a significantly higher rate of locoregional failure. Ben-Josef et al. JCO 2010
- 197. Local Failure Ben-Josef et al. JCO 2010
- 198. Overall Survival Ben-Josef et al. JCO 2010
- 199. Adjuvant therapy after APR • No data to suggest indications of adjuvant therapy after surgery. • Generally extrapolated from other sites: - T3/T4 - Node +ve - Margin +ve - High risk features: poor differentiation, LVSI, ulcerative lesions, male sex, HIV+/MSM
- 200. Summary • Definitive chemoradiation (EBRT 45-54 Gy + 5FU/MMC) is the management of choice for anal SCC. - Treatment breaks to be avoided as far as possible. • APR reserved for salvaging treatment failures/ recurrences. • No benefit with neoadjuvant or adjuvant chemotherapy. • No benefit with a higher dose boost.
- 202. Patient Positioning & Simulation • Supine with arms across the chest or prone in a belly board • If using the prone setup for primary fields, may consider supine positioning for the boost • Due to high rates of moist skin desquamation • Contrast agents and markers - Oral contrast to delineate small bowel - Barium enema - IV contrast to delineate the tumor and LN. - Anal marker to delineate the anus. - Wire to involved inguinal LN.
- 203. Field Design: Principles • Large AP/PA fields that include the inguinal lymph nodes will deliver the full radiation dose to the femoral head and neck. • Patients treated in this way may be at risk for radiation-induced fracture. • Lateral inguinal lymph nodes generally treated only through anterior fields. The posterior field is designed to treat only the primary tumor and the pelvic lymph nodes. • Minimize the dose to the femoral head and necks. • If necessary, electron fields are used to supplement the dose to the lateral superficial inguinal nodes that are not included in the posterior photon field.
- 204. Whole Pelvic Field AP-PA Fields • Superior border - L5/S1 junction for the first 30.6 -36 Gy. - Lowered to lower margin of SIJ / 3 cm above the cranial extent of disease to 45 Gy. • Inferior border: > 2-3 cm below the anal verge or the inferior extent of the primary tumor - whichever is lower. • Lateral borders: - PA field: 1.5-2 cm lateral to widest portion of the pelvic brim. - AP field to include inguinal nodes till ~ 36 Gy, matched to PA thereafter. - Clinically positive nodes should be outlined with radio-opaque wire. • Generally includes the entire femoral heads. Conventional field borders
- 205. • Wide anterior (A) and narrow posterior (B) photon fields. Electron fields are used to dose the lateral inguinal nodes not included in the narrow posterior photon field (C and D). • The medial border of each electron field is placed at the posterior photon field’s lateral exit point on the anterior abdominal wall.
- 206. Anteroposterior treatment portal film. Nigro et al, Cancer 1983.
- 207. Conventional field borders • Anterior border: Posterior margin of the symphysis pubis (to treat only the internal iliac nodes). • Posterior border: 1-1.5 cm behind the anterior body sacral margin • Superior/ Inferior borders: Same as AP-PA fields. Lateral Fields - Not always employed; may be used as part of a 3 field design or a 4 field box
- 208. Perineal Boost • Prone or supine; prone usually difficult due to skin toxicity from Phase I • Two,3 or 4 field design may be employed • 2.5-3 cm margin to be taken on gross disease (AP and lateral) - Clinical + radiological
- 209. Dose / Fractionation • Phase 1: 36 Gy - Whole Pelvic Fields (wide AP + narrow PA) • Phase 2: 9 Gy (36 → 45) - Whole pelvis, with AP fields matched to PA fields - May lower the cranial border to lower end of SIJ • Phase 3: 5.4 - 9 Gy* (45 → 50.4 - 54) - Boost additional 5.4 to 9 Gy depending on T/N size & clinical response. - May be omitted for early lesions (T1/T2, node negative). • Inguinal fields: 9-18 Gy (photon or electron) - Prescription depth ideally CT defined/3-4cms * no evidence in favour of high dose boost
- 210. Conformal Radiotherapy Target Volume Definitions • GTV: Primary tumor + positive LN on planning CT (>1 cm short axis diameter). • CTV - Nodes at risk include common iliac, external iliac, internal iliac, presacral, perianal, and inguinal. - CTV_A = 2.5 cm expansion on primary tumor and 1 cm expansion on involved nodes - CTV_B (Whole Pelvis CTV) = CTV_A + Nodal areas at risk • PTV: Setup margins on CTV as per institutional protocol
- 211. Planning Objectives • 95% of the PTV should receive at least 95% of the prescription dose. • Dose limitations • Small bowel: V40-45Gy < 200 cc • Femoral Head: D50 < 30-35 Gy • Bladder: D50 < 35-40 Gy • Marrow: V10 < 90%, V20 < 80% • External genitalia: D50 < 20 Gy, D5 < 40 Gy
- 213. Brachytherapy • In most series, patients received 30 to 55 Gy of EBRT + 5-FU/MM-C or cisplatin followed by a 15 to 25 Gy boost with Ir192 afterloading catheters. • Delivered intraluminally, through a rectal applicator that has direct contact with the tumor, or through interstitial catheters. • Papillon and Montbarbon reported a series of 276 patients of whom 222 underwent CRT (30 - 42 Gy) → 2 month break → 15-20 Gy Ir 192 interstitial template based boost. • Followed for at least 5 years, 65% DFS was noted, with a 7.4% failure rate in the inguinal nodes. • Other authors have also reported encouraging results; however it is yet to be compared directly against an EBRT boost.2 1. Papillon et al. Dis Colon Rectum. 1987 2. Niehoff P, Kovacs G. Gastrointest Oncol 2014
- 214. Salvage Therapy for Local Recurrence • Most patients undergo an APR and a permanent colostomy. • Local structures invasion requires multivisceral resection to achieve negative margins. • Reported 5 year results: OS 30% - 64%, DFS 30% - 40%. • The most important prognostic factor of survival after resection is margin status, and patients with negative margins (R0) have up to >50% long term survival.
- 215. AIN (Anal Intraepithelial Neoplasia) • Transanal excision. • Microinvasive disease: if incompletely excised, reexcision is required once the surgical site is fully healed. Topical options • Imiquimod, a nucleoside analog has local proinflammatory and antiviral properties and has shown good results in the eradication of AIN III. • Fluorouracil has been shown to have high efficacy but causes significant side effects, such as burning, irritation, and pain. Other options • Infrared coagulation, • Cryotherapy, • CO2 laser ablation, and 5-ALA/photodynamic therapy. • High rates of recurrence, esp. in high risk groups.
- 216. Anal Margin Cancers T1N0 > T2 Node +ve / Advanced • WLE with 1cm margin • If positive margins: • Re-excision • If re-excision not feasible, RT + CT • Chemoradiation • Combined modality treatment • APR + adjuvant treatment
- 217. Squamous Cell Cancer Of The Anal Margin • Papillon and Chassard reported on 8 patients with T1/T2 lesions treated with either radium implant brachytherapy alone (6 patients) or in combination with external- beam radiation (2 patients) . • Local control rate was 100%. An additional 36 patients were treated with EBRT + 5-FU/MMC. Cure rate for T1, T2, and T3 lesions were 100% , 84% & 50%. • Cummings compared radiation alone versus chemoradiation retrospectively in 29 patients • Median 7 yr follow-up, the local control rate was 64% for the radiation group and 88% for the chemoradiation group. The local control rate was inversely associated with larger cancers (T1-T2, 100 % ; T3 5-10 cm, 70%; T3 > 10 cm, 40%).
- 218. Adenocarcinoma Of The Anal Canal • Extension of rectal cancer into the anal canal far commoner than de novo adenocarcinoma of the anal canal. - May arise from anal glands or anal fistulae. - Occasionally in patients with Inflammatory Bowel Disease who have ileal pouch-anal anastomosis. • APR should be offered for early-stage disease. • Locally advanced disease (T3+ or N+): Combined Modality
- 219. Melanoma Of The Anorectal Region • The 5 -year OS rate is generally less than 20% . The initial stage at presentation largely determines OS. • Ross et al from the M. D. Anderson Cancer Center reviewed a series of 32 patients with melanoma treated with either APR or local resection. • Local recurrence was lower in the APR group (29% APR vs 5.8%). However, there was no difference in OS (19.5 months for APR; 18.9 months for local resection). • Given available data, local excision advisable if adequate margins possible; else APR should be done. - Adjuvant therapy to be decided along the lines of melanoma. Ross et al. Arch Surg, 1990 Singer M, Mutch MG. Clin Colon Rectal Surg, 2006
- 220. Management of advanced/metastatic disease • Approximately 10%–20% patients • Poor prognosis; 2 year survival less than 25%. • No consensus on the standard chemotherapy treatment; NCCN recommends combination chemotherapy with CDDP + 5FU as 1st line. • Other options: • Taxanes, carboplatin, doxorubicin, irinotecan (alone or in combination). • Cetuximab (KRAS wild) NCCN 2017
- 221. Surveillance
- 222. Thank You
