IB Chemistry on Resonance, Delocalization and Formal Charges
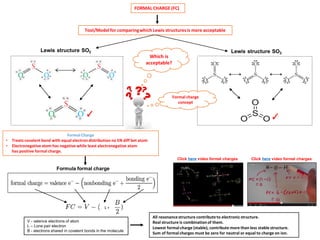
- 1. FORMAL CHARGE (FC) Tool/Model for comparing which Lewis structures is more acceptable Lewis structure SO2 Which is acceptable? Lewis structure SO3 Formal Charge •Treats covalent bond with equal electron distribution no EN diff bet atom •Electronegative atom has negative while least electronegative atom has positive formal charge. Formula formal charge Click herevideo formal charges Click herevideo formal charges V -valence electronsof atom L–Lone pair electron B -electrons shared incovalent bonds in the molecule ✓ ✓ All resonance structure contribute to electronic structure. Real structure is combination of them. Lowest formal charge (stable), contribute more than less stable structure. Sum of formal charges must be zero for neutral or equal to charge on ion. L+ Formal charge concept
- 2. FORMAL CHARGE (FC) Formal Charge •Tool/Model for comparing which Lewis structures is more acceptable •Treats covalent bond with equal electron distribution no electronegativity differences bet atom •Electronegative atom has negative while least electronegative atom has positive formal charge Formula formal charge V -valence electronsof atom L –lone pair electron B –bonding electron molecule ✓ Formal charge carbon dioxide formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 4 B-Bonding electron O = 4 L+ FC = 6 –(4+2) = 0 formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 4 B-Bonding electron O = 4 FC = 6 –(4+2) = 0 formal charge for C V-Valence electron C = 4 L -Lone pair electron C = 0 B -Bonding electron C = 8 FC = 4 –(0+4) = 0 ✓ Lowest formal charge is preferred L+ L+ L+
- 3. Formal charge carbon dioxide Formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 4 B-Bonding electron O = 4 FC = 6 –(4+2) = 0 Formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 4 B-Bonding electron O = 4 FC = 6 –(4+2) = 0 Formal charge for C V-Valence electron C = 4 L -Lone pair electron C = 0 B -Bonding electron C = 8 FC = 4 –(0+4) = 0 ✓ Lowest formal charge is preferred Different Lewis structures for CO2 Which is acceptable? 0 0 0 0 -1 +1 -1 +2 -1 0 -2 +2 Lowest formal charge is preferred ✓ Click herevideo CO2 Lowest formal charge –more stable -contribute more to diff resonance structures. Sum of formal charges must be zero for neutral or equal to charge on ion. L+ L+ L+
- 4. Formal charge dinitrogen oxide Formal charge for N Formal charge for N Formal charge for O V-Valence electron N = 5 L-Lone pair electron N = 2 B-Bonding electron N = 6 V-Valence electron N = 5 L-Lone pair electron N = 0 B-Bonding electron N = 8 V-Valence electron O = 6 L-Lone pair electron O = 6 B-Bonding electron O = 2 FC = 5 –(2+3) = 0 FC = 5 –(0+4) = +1 FC = 6 –(6+1) = -1 +1 0 -1 Different Lewis structures for N2O -2 +1 +1 +1 -1 0 0 +1 -1 -1 +2 -1 -2 +2 0 Lowest formal charge is preferred ✓ Click hereto view video Which is acceptable? Lowest formal charge is preferred ✓ All resonance structure contribute to electronic structure. Real structure is combination of them. Lowest formal charge (stable), contribute more than less stable structure. Sum of formal charges must be zero for neutral or equal to charge on ion. L+ L+ L+
- 5. Delocalization of electrons Resonance • Describing delocalization of electrons within a molecule/polyatomic ion where bonding cannot be express by ONE single Lewis structure •Delocalization of π bond – π electrons spread over more than 2 nuclei •π electrons are shared •π electrons spread – more stable Resonance structures carbonate ion 2 3 CO resonance structure 1 resonance structure 2 resonance structure 3 Resonance hybrid • All bonds CO3 2- are identical in length and strength • Hybrid of 3 resonance structures • Negative charge equally distributed over all oxygen • No O-O (single) or O=O (double) bonds found • Only O -----O bond • Intermediate in character bet single and double bond • Bond Order = 3 1 1 Carbonate Ion • charge 2- delocalized into 2/3- • lower charge – more stable Click here on video carbonate C
- 6. All resonance structure contribute to electronic structure. Real structure is combination of them. Lowest formal charge (stable), contribute more than less stable structure. Sum of formal charges must be zero for neutral or equal to charge on ion. FORMAL CHARGE (FC) Formal Charge •Tool/Model for comparing which Lewis structures is more acceptable •Treats covalent bond with equal electron distribution no electronegativity differences bet atom •Electronegative atom has negative while least electronegative atom has positive formal charge Formula formal charge V -valence electronsof atom L –lone pair electron B –bonding electron molecule ✓ Formal charge carbonate ion formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 6 B-Bonding electron O = 2 L+ FC = 6 –(6 +1) = -1 formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 4 B-Bonding electron O = 4 FC = 6 –(4+2) = 0 formal charge for C V-Valence electron C = 4 L -Lone pair electron C = 0 B -Bonding electron C = 8 FC = 4 –(0+4) = 0 -1 -1 0 0 ✓ L+ L+ L+
- 7. Delocalization of electrons Resonance structures nitrate ion 3 NO resonance structure 1 resonance structure 2 resonance structure 3 resonance hybrid • All bonds NO3 - are identical in length and strength • Hybrid of 3 resonance structures • Negative charge equally distributed over all oxygen • No N-O (single) or N=O (double) bonds found • Only N -----O bond • Intermediate in character bet single and double bond • Bond Order = 3 1 1 Nitrate Ion charge of -1 delocalized into 1/3- lower charge – more stable Click here to view video Resonance • Describing delocalization of electrons within a molecule/polyatomic ion where bonding cannot be express by ONE single Lewis structure •Delocalization of π bond – π electrons spread over more than 2 nuclei •π electrons are shared •π electrons spread – more stable 1/3 1/3 1/3
- 8. All resonance structure contribute to electronic structure. Real structure is combination of them. Lowest formal charge (stable), contribute more than less stable structure. Sum of formal charges must be zero for neutral or equal to charge on ion. FORMAL CHARGE (FC) Formal Charge •Tool/Model for comparing which Lewis structures is more acceptable •Treats covalent bond with equal electron distribution no electronegativity differences bet atom •Electronegative atom has negative while least electronegative atom has positive formal charge Formula formal charge V -valence electronsof atom L –lone pair electron B –bonding electron molecule ✓ Formal charge nitrate ion formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 6 B-Bonding electron O = 2 L+ FC = 6 –(6 +1) = -1 formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 4 B-Bonding electron O = 4 FC = 6 –(4+2) = 0 formal charge for N V-Valence electron N = 5 L -Lone pair electron N = 0 B -Bonding electron N = 8 FC = 5 –(0+4) = +1 -1 -1 +1 0 ✓ L+ L+ L+
- 9. Delocalization of electrons Resonance structures nitrite ion 2 NO resonance structure 1 resonance structure 2 resonance hybrid • All bonds NO2 - are identical in length and strength • Hybrid of 2 resonance structures • Negative charge equally distributed over all oxygen • NO N-O (single) or N=O (double) bonds found • Only N ----- O bond • Intermediate in character bet single and double bond • Bond Order = 2 1 1 Nitrite Ion charge of -1 delocalized into 1/2- lower charge – more stable Resonance • Describing delocalization of electrons within a molecule/polyatomic ion where bonding cannot be express by ONE single Lewis structure •Delocalization of π bond – π electrons spread over more than 2 nuclei •π electrons are shared •π electrons spread – more stable Click here video nitrite
- 10. All resonance structure contribute to electronic structure. Real structure is combination of them. Lowest formal charge (stable), contribute more than less stable structure. Sum of formal charges must be zero for neutral or equal to charge on ion. FORMAL CHARGE (FC) Formal Charge •Tool/Model for comparing which Lewis structures is more acceptable •Treats covalent bond with equal electron distribution no electronegativity differences bet atom •Electronegative atom has negative while least electronegative atom has positive formal charge Formula formal charge V -valence electronsof atom L –lone pair electron B –bonding electron molecule ✓ Formal charge nitrite ion formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 4 B-Bonding electron O = 4 L+ FC = 6 –(4 +2) = 0 formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 6 B-Bonding electron O = 2 FC = 6 –(6+1) = -1 formal charge for N V-Valence electron N = 5 L -Lone pair electron N = 2 B -Bonding electron N = 6 FC = 5 –(2+3) = 0 -1 0 0 ✓ L+ L+ L+
- 11. Delocalization of electrons Resonance structures sulfur dioxide 2 SO resonance structure 1 resonance structure 2 • All SO2 bonds are identical in length and strength • Hybrid of 2 resonance structures • Negative charge equally distributed over all oxygen • NO S-O (single) or S=O (double) bonds found • Only S -----O bond • Intermediate in character bet single and double bond • Bond Order = 2 1 1 Sulfur Dioxide Click here to view S Resonance • Describing delocalization of electrons within a molecule/polyatomic ion where bonding cannot be express by ONE single Lewis structure •Delocalization of π bond – π electrons spread over more than 2 nuclei •π electrons are shared •π electrons spread – more stable resonance structure 3 How about structure 3? resonance hybrid
- 12. FORMAL CHARGE (FC) Formal Charge •Tool/Model for comparing which Lewis structures is more acceptable •Treats covalent bond with equal electron distribution no electronegativity differences bet atom •Electronegative atom has negative while least electronegative atom has positive formal charge Formula formal charge V -valence electronsof atom L –lone pair electron B –bonding electron molecule ✓ Formal charge sulfur dioxide formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 4 B-Bonding electron O = 4 L+ FC = 6 –(4 +2) = 0 formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 4 B-Bonding electron O = 4 FC = 6 –(4+2) = 0 formal charge for S V-Valence electron S = 6 L -Lone pair electron S = 2 B -Bonding electron S = 8 FC = 6 –(2+4) = 0 All resonance structure contribute to electronic structure. Real structure is combination of them. Lowest formal charge, contribute more than less stable structure. Sum of formal charges must be zero for neutral or equal to charge on ion. ✓ 0 0 0 ✓ L+ L+ L+
- 13. Formal charge Sulfur dioxide Formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 4 B-Bonding electron O = 4 FC = 6 –(4 +2) = 0 Formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 4 B-Bonding electron O = 4 FC = 6 –(4+2) = 0 Formal charge for S V-Valence electron S = 6 L -Lone pair electron S = 2 B -Bonding electron S = 8 FC = 6 –(2+4) = 0 ✓ Lowest formal charge is preferred Different Lewis structures for SO2 Which is acceptable? Lowest formal charge is preferred ✓ All resonance structure contribute to electronic structure. Real structure is combination of them. Lowest formal charge (stable) contribute more than less stable structure. Sum of formal charges must be zero for neutral or equal to charge on ion. 0 0 0 0 0 0 0 +1 -1 0 +1 -1 Click hereto view L+ L+ L+
- 14. Delocalization of electrons Resonance structure cyanate resonance structure 1 resonance structure 2 Cyanate ion Resonance • Describing delocalization of electrons within a molecule/polyatomic ion where bonding cannot be express by ONE single Lewis structure •Delocalization of π bond – π electrons spread over more than 2 nuclei •π electrons are shared •π electrons spread – more stable resonance structure 3 Which structure is acceptable ? -1 0 0 0 0 -1 +1 0 -2 Contribute the MOST Negative formal charge located on more electronegative O atom is more stable than one located on a less electronegative N atom Lowest formal charge is preferred ✓ 0 0 -1 ✓ Contribute the least High formal charge/unstable ✓ χ χ Contribute less Negative formal charge on less electronegative N atom - NCO Click here to view
- 15. Delocalization of electrons Xenon trioxide Resonance •Describing delocalization of electrons within a molecule/polyatomic ion where bonding cannot be express by ONE single Lewis structure •Delocalization of π bond – π electrons spread over more than 2 nuclei •π electrons are shared •π electrons spread – more stable Which structure is acceptable ? 3 XeO Click here to view Different resonance structure for XeO3 ✓ Lowest formal charge is preferred formal charge for O V- Valence electron O = 6 L- Lone pair electron O = 4 B- Bonding electron O = 4 FC = 6 – (4 +2) = 0 formal charge for Xe formal charge for O V- Valence electron Xe = 8 L- Lone pair electron Xe = 2 B- Bonding electron Xe = 12 V- Valence electron O = 6 L- Lone pair electron O = 4 B- Bonding electron O = 4 FC = 8 – (2 +6) = 0 FC = 6 – (4 +2) = 0 0 0 0 0 L + L + L +
- 16. Delocalization of electrons Resonance structures sulfur trioxide resonance structure 1 resonance structure 2 • All SO3 bonds are identical in length and strength • Hybrid of 3 resonance structures • NO S-O (single) or S=O (double) bonds found • Only S -----O bond • Intermediate in character bet single and double bond • Bond Order = 3 1 1 Sulfur Trioxide 3 SO resonance structure 3 S 120 Click here to view video Resonance • Describing delocalization of electrons within a molecule/polyatomic ion where bonding cannot be express by ONE single Lewis structure •Delocalization of π bond – π electrons spread over more than 2 nuclei •π electrons are shared •π electrons spread – more stable resonance structure 4 How about structure 4 ? resonance hybrid
- 17. Formal charge Sulfur Trioxide Formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 4 B-Bonding electron O = 4 FC = 6 –(4 +2) = 0 Formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 4 B-Bonding electron O = 4 FC = 6 –(4+2) = 0 Formal charge for S V-Valence electron S = 6 L -Lone pair electron S = 0 B -Bonding electron S = 12 FC = 6 –(0+6) = 0 ✓ Lowest formal charge is preferred Different Lewis structures for SO3 Which is acceptable? Lowest formal charge is preferred ✓ All resonance structure contribute to electronic structure. Real structure is combination of them. Lowest formal charge (stable), contribute more than less stable structure. Sum of formal charges must be zero for neutral or equal to charge on ion. 0 0 0 0 0 0 0 0 0 -1 -1 +2 0 -1 -1 +2 0 +2 -1 -1 L+ L+ L+
- 18. Delocalization of electrons Resonance structures methanoate resonance structure 1 resonance structure 2 • All CO bonds are identical in length and strength • Hybrid of 2 resonance structures • NO C-O (single) or C=O (double) bonds found • Only C ----- O bond • Intermediate character bet single and double bond • Bond Order = 2 1 1 Methanoate ion HCOO Resonance • Describing delocalization of electrons within a molecule/polyatomic ion where bonding cannot be express by ONE single Lewis structure •Delocalization of π bond – π electrons spread over more than 2 nuclei •π electrons are shared •π electrons spread – more stable Click here to view resonance hybrid Click here to view Resonance structures ethanoate Ethanoate ion CH COO 3 resonance structure 1 resonance structure 2 resonance hybrid H H CH3
- 19. FORMAL CHARGE (FC) Formal Charge •Tool/Model for comparing which Lewis structures is more acceptable •Treats covalent bond with equal electron distribution no electronegativity differences bet atom •Electronegative atom has negative while least electronegative atom has positive formal charge Formula formal charge V -valence electronsof atom L –lone pair electron B –bonding electron molecule ✓ Formal charge methanoate ion formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 6 B-Bonding electron O = 2 L+ FC = 6 –(6 +1) = -1 formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 4 B-Bonding electron O = 4 FC = 6 –(4+2) = 0 formal charge for C V-Valence electron C = 4 L -Lone pair electron C = 0 B -Bonding electron C = 8 FC = 4 –(0+4) = 0 All resonance structure contribute to electronic structure. Real structure is combination of them. Lowest formal charge (stable), contribute more than less stable structure. Sum of formal charges must be zero for neutral or equal to charge on ion. ✓ 0 0 -1 0 L+ L+ L+
- 20. Delocalization of electrons Resonance structures thiocyanate resonance structure 1 resonance structure 2 Thiocyanate ion SCN Click here to view video Resonance • Describing delocalization of electrons within a molecule/polyatomic ion where bonding cannot be express by ONE single Lewis structure •Delocalization of π bond – π electrons spread over more than 2 nuclei •π electrons are shared •π electrons spread – more stable resonance structure 3 Which is acceptable structure? -1 0 0 0 0 -1 +1 0 -2 Contribute the MOST Negative formal charge located on more electronegative N atom is more stable than one located on a less electronegative S atom Lowest formal charge is preferred ✓ 0 0 -1 ✓ Contribute the least High formal charge/unstable ✓ χ χ Contribute the less Negative formal charge on less electronegative S atom
- 21. Formal charge thiocyanate ion Formal charge for S Formal charge for C Formal charge for N V-Valence electron S = 6 L-Lone pair electron S = 4 B-Bonding electron S = 4 V-Valence electron C = 4 L-Lone pair electron C = 0 B-Bonding electron C = 8 V-Valence electron N = 5 L-Lone pair electron N = 4 B-Bonding electron N = 4 FC = 6 –(4+2) = 0 FC = 4 –(0+4) = 0 FC = 5 –(4+2) = -1 0 0 -1 Different Lewis structures for SCN- 0 -1 0 Lowest formal charge is preferred ✓ Which is acceptable? Lowest formal charge is preferred ✓ All resonance structure contribute to electronic structure. Real structure is combination of them. Lowest formal charge (stable), contribute more than less stable structure. Sum of formal charges must be zero for neutral or equal to charge on ion. 0 0 -1 +1 0 -2 Click hereto view video L+ L+ L+
- 22. Delocalization of electrons Resonance structures ozone resonance structure 1 resonance structure 2 resonance hybrid • All bonds O-O are identical in length and strength • Hybrid of 2 resonance structures • NOO-O (single) or O=O (double) bonds found • Only O -----O bond • Intermediate in character bet single and double bond • Bond Order = 2 1 1 Ozone 3 O Click here on video ozone Resonance • Describing delocalization of electrons within a molecule/polyatomic ion where bonding cannot be express by ONE single Lewis structure •Delocalization of π bond – π electrons spread over more than 2 nuclei •π electrons are shared •π electrons spread – more stable • Pale blue gas, polar, dimagnetic • Oxidizing agent • Potent respiratory hazard and pollutant at ground level • Beneficial prevent UV B/C from reaching Earth surface • Highest ozone level in stratosphere,(10 km and 50 km) UV radiation Ozone at stratosphere strongest radiation 3 O
- 23. FORMAL CHARGE (FC) Formal Charge •Tool/Model for comparing which Lewis structures is more acceptable •Treats covalent bond with equal electron distribution no electronegativity differences bet atom •Electronegative atom has negative while least electronegative atom has positive formal charge Formula formal charge V -valence electronsof atom L –lone pair electron B –bonding electron molecule ✓ Formal charge sulfur dioxide formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 4 B-Bonding electron O = 4 L+ FC = 6 –(4 +2) = 0 formal charge for O V-Valence electron O = 6 L-Lone pair electron O = 6 B-Bonding electron O = 2 FC = 6 –(6+1) = -1 formal charge for O V-Valence electron O = 6 L -Lone pair electron O = 2 B -Bonding electron O = 6 FC = 6 –(2+3) = +1 All resonance structure contribute to electronic structure. Real structure is combination of them. Lowest formal charge (stable), contribute more than less stable structure. Sum of formal charges must be zero for neutral or equal to charge on ion. ✓ 0 +1 -1 ✓ L+ L+ L+
- 24. Ozone Good and Bad Good Side Bad Side Ozone in Strastophere •blocks UV B + C Ozone in Troposphere act as •Greenhouse gas Ozone in ground level act as •Pollutant •Photochemical Click hereon ozone depletion chemicals (phaseout) Why ozone able to absorb UV B and UV C? Breakdown of ozone –High UV radiation –Skin cancer -DNA mutation Ozone depletion UV Exposure
