IB Chemistry on Polarity, Hydrogen Bonding and Van Der Waals forces
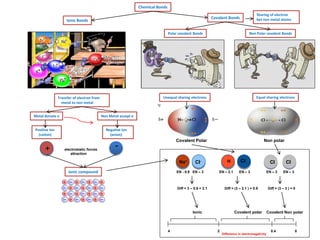
- 1. Chemical Bonds Ionic Bonds Transfer of electron from metal to non metal Metal donate e Non Metal accept e Positive ion (cation) Negative ion (anion) Ionic compound Covalent Bonds Sharing of electron bet non metal atoms Equal sharing electrons + -electrostatic forces attraction 4 0.4 0 Difference in electronegativity 2 EN - 0.9 EN – 3 Diff = (3 – 3 ) = 0 H EN – 2.1 Diff = 3 – 0.9 = 2.1 Polar covalent Bonds Non Polar covalent Bonds Unequal sharing electrons Covalent Polar Non polar CI CI EN – 3 Covalent Non polar CI Covalent polarIonic EN – 3 Diff = (3 – 2.1 ) = 0.9 Na+ CI- EN – 3
- 2. Shared electron cloud closer to O Electronegativity Electronegativity (EN) •Tendency of atom to attract/pull shared/bonding electron to itself •EN value higher – pull/attract electron higher (EN value from 0.7 – 4) Electronegativity • EN increase up a Group • EN increase across a Period H 2.2 Li Be B C N O F CI Br I 1 1.6 2 2.6 3 3.4 4 Electronegativity values N, O, F have high EN value 3.2 3 2.7 Molecule Diff in EN Polarity H - F (4.0 – 2.2) = 1.8 Most polar H - CI (3.2 – 2.2) = 1 H - Br (3.0 – 2.2) = 0.8 H - I (2.7 – 2.2) = 0.5 Least polar Polarity Shape Diff in EN Symmetrical Asymmetrical Bond polarity cancel out each other Polar bonds – molecule NON POLAR Bond polarity cancel out each other Polar bonds – molecule POLAR Lewis structure VSEPR Geometry 1 4 ECC 3 bond pair 1 lone pair .. N H H H Polarity 2 3 4 Polar ✓✗
- 3. ✗ In presence of electric field Separation of charges Unequal distribution electron due to diff EN value shared electron closer to Oshared electron closer to F Covalent Bonds Polar covalent Bonds Non Polar covalent Bond Equal sharing electronUnequal sharing electron Sharing of electron Formation electric dipole Partial +/- Dipole moment towards O Partial + ( δ+) Partial – (δ−) Net dipole moment Molecule is polar (dipole) Net Dipole moment Measured in Debye Turning force / Dipole moment = Force x DistancePolar covalent Bonds + - O III C δ+ δ- Turning force – dipole moment + - O II C II O δ+ δ- δ- No Turning force – No dipole moment ✓ Molecule polar ✓ O O
- 4. Polarity Shape Asymmetrical Polar bond Polarity dont cancel (ASYMMETRICAL) Net dipole moment Molecule POLAR Polar bond Polarity cancel (SYMMETRICAL) NO net dipole moment Molecule NON POLAR Shape Symmetrical Polar bonds CI Polar bonds δ- δ+ δ+ δ+ δ- δ- δ- δ- δ- δ- Bond polarity don’t cancel Bond polarity cancel H Net Dipole moment No Net Dipole moment ✗ Asymmetrical Symmetrical δ-δ+ Polar bonds Bond polarity don’t cancel Net Dipole moment C O Polar bond Polarity dont cancel (ASYMMETRICAL) Net dipole moment Molecule POLAR δ- δ- Polar bonds Bond polarity cancel No Net Dipole moment Polar bond Polarity cancel (SYMMETRICAL) NO dipole moment Molecule NON POLAR ✗ ✗✓ ✓ I
- 5. Bonding Forces Bonding Forces within molecule Bonding Forces bet molecule Intermolecular force bet molecule (IMF)Ionic bond Covalent bond Metallic bond Permanent dipole IonsMolecules/NOT ions Ion dipole forces Polar Non Polar Hydrogen bonding Temporary dipole (instantaneously induced dipole) London dispersion forces Van Der Waals’ Forces attraction Polar molecule (dipole – dipole attraction) _ _ _ Attraction bet ions with polar molecules Dipole/dipole attraction Dipole/dipole attraction (involving H atom attach to N,O F) Hydrogen bonding (dipole – dipole attraction) Forces bet molecule
- 6. Permanent dipole Polar Non Polar Temporary dipole (instantaneously induced dipole) London dispersion forces Van Der Waals’ Forces attraction Polar molecule (dipole – dipole attraction) Polar molecules due to diff in EN values Dipole/dipole interaction Electrostatic forces attraction bet molecules Dipole/dipole attraction Dipole/dipole attraction Hydrogen bonding (dipole – dipole attraction) H atom bond to electronegative atom, N, O, F Partial H+ attracted to lone pair electron on N, O, F Electrostatic force attraction bet molecules involve H Intermolecular force bet molecule (IMF) Non Polar molecule (Induced dipole attraction) Random movement /distribution of electron Instantaneous negative charge on atom Induced a temporary dipole separation Electrostatic forces attraction bet molecules Non polar molecules Polar molecules Polar molecules Forces bet molecule Molecules
- 7. Hydrogen bonding (dipole – dipole attraction) H atom bond to electronegative atom, N, O, F Partial H+ attracted to lone pair electron on N, O, F Electrostatic force attraction bet molecules involve H Permanent dipole Polar Non Polar Temporary dipole (instantaneously induced dipole) London dispersion forces Van Der Waals’ Forces attraction Polar molecule Polar molecules due to diff in EN values Dipole/dipole interaction Electrostatic forces attraction bet molecules Dipole/dipole attraction Dipole/dipole attraction Molecules Non Polar molecule (Induced dipole attraction) Random movement /distribution of electron Instantaneous negative charge on atom Induced a temporary dipole separation Instantaneous dipole in one atom induce a dipole in its neighbor Electrostatic forces attraction bet molecules Non polar molecules Polar molecules Polar molecules Requirement for H bonding •H atom bonded to N, O, F •N, O, F - highly electronegative/ small size •Attract electron close to itself – Polarised H+ •N---H, O—H, F—H bonds VERY POLAR •Very polar H+ attract closely to lone pair on N, O, F N ---- H O ---- H F ---- H δ- δ- δ-δ+ δ-+ δ+
- 8. Types of forces/Bonding Factors affecting VDF forcesIntermolecular force bet molecule (IMF) Interaction Strength Boiling Point/C Covalent Strongest High Ionic Strong High Ion dipole Strong HIgh Dipole- dipole (H bond) Moderate High Dipole – dipole Weak Low Temporary induce dipole (London dispersion) Weakest Low Dipole – dipole attraction Induced – dipole attraction London dispersion forces Polar Non Polar All molecules have London dispersion forces due to uneven distribution of electron cloud - - - - - - - - - - - - - - - δ+δ- London dispersion forces RMM/Size Surface Area London dispersion forces Van Der Waals’ Forces attraction N N F F RMM – 38 RMM – 28 Size/ RMM increase Number electrons increase Temporary induced dipole increase Van Der Waals bet molecule increase RMM same Surface area increase Temporary induced dipole increase Van Der Waals bet molecules increase RMM – 72 RMM – 72 Pentane (C5H12) Factor affecting b/p of molecules Temporary dipole attraction London dispersion force Permanent dipole attraction Dipole/dipole attraction Hydrogen bonding
- 9. Factors affecting VDF forcesFactor affecting b/p of molecules RMM/Size Surface Area N N F F RMM – 38 RMM – 28 Size/ RMM increase Number electrons increase Temporary induced dipole increase Van Der Waals bet molecule increase RMM same Surface area increase Temporary induced dipole increase Van Der Waals bet molecules increase RMM – 72 RMM – 72 Pentane (C5H12) Temporary dipole attraction London dispersion force Permanent dipole attraction Dipole/dipole attraction Hydrogen bonding H2 N2 CI2 H2O RMM 2 28 71 18 Boiling Point/C -252 -196 -34 100 Forces London force London force London force London force Dipole/dipole Hydrogen bonding - - -- -- H2 London forces N2 London forces CI2 London forces H2O London forces Dipole/dipole Hydrogen bond RMM increase - London force/VDF increase – boiling point increase - - - - -- - - -- - - - - - - Hydrogen bondingHydrogen bonding RMM lowest - boiling point highest - due to hydrogen bonding
- 10. HCI HBr HI HF RMM 36.5 81 128 20 Boiling Point/C -85 -66 -35 19.5 Forces London force/VDF London force/VDF London force/VDF London force/VDF Dipole/dipole Hydrogen bond RMM increase - London force/VDF increase – boiling point increase Which liquid has higher boiling point? H H H H H H DNA Br Br Br I I I Hydrogen bonding RMM lowest - boiling point highest - due to hydrogen bonding Br2 ICI RMM 162 162 B/p/C 58 97 Forces London force/VDF London force/VDF Dipole/dipole Which liquid has higher boiling point? - - - - - - Br2 London forces bet molecules Br Br Br Br I I ICI CI CI + + +- - - ICI London forces + Dipole –dipole attraction Hydrogen Bond bet nitrogenous base
- 11. CH3CH2OH CH3CH2CH2OH CH3COOH C2H5-O-C2H5 RMM 46 60 60 74 Boiling Point/C 78 97 118 34 Forces London H2 bond London H2 bond London H2 bond London RMM - London force – boiling point Which liquid has higher boiling point? Stronger Hydrogen bond – boiling point CH3COOH boiling point higher C=O (carbonyl) – electron withdrawing gp withdraw electron from OH gp O-H gp more polarised stronger H2 bond Hydrogen bond Hydrogen bond ✕ C - O - H = o C3H8 CH3CHO CH3CH2OH RMM 44 44 46 Boiling Point/C -42 20.2 118 Forces London London Dipole/dipole London Dipole/dipole H2 bond RMM highest No Hydrogen Bond Which liquid has higher boiling point? ....... .......... London forces London forces + Dipole/dipole London forces + Dipole/dipole + Hydrogen Bond ✕ Hydrogen bond
- 12. Why 2 Nitrophenol has lower b/p than 4 nitrophenol? Molecule symmetrical Bond polarity cancel No net dipole moment Molecule NON POLAR C6H5NO3 ( 2 nitrophenol) C6H5NO3 ( 4 nitrophenol) RMM 139 139 Boiling Point/C 216 279 Forces London Intramolecular H2 bond London Intermolecular H2 bond Which NCI3 is polar but BCI3 non polar? ... … Intramolecular H2 bond Non polar …… ✕ More intramolecular H2 bond Lack intermolecular H2 bond Intermolecular H2 bond ✓ ✓ More intermolecular H2 bond Lack intramolecular H2 bond BCI3 NCI3 RMM 117 120 Boiling Point/C 13 71 Forces London London Dipole/dipole …... Non polar ... δ- δ- δ- δ-+ polar polar …..... Molecule asymmetrical Bond polarity does not cancel NET dipole moment Molecule POLAR ✓Dipole dipole
- 13. Trans isomer – CI opposite side Bond polarity cancel NO Net dipole moment / NON POLAR Intermolecular forces weaker Molecule in linear shape Able to pack closely together Greater surface area for interaction Intermolecular forces stronger Polarity for Cis/Trans Geometrical Isomers Molecule in kink/bend shape Unable to pack closely together Less surface area for interaction Intermolecular forces weaker Cis dichloroethene Trans dichloroethene RMM 96 96 Melting Point/C -80 -50 Boiling Point/C 60 48 ... δ- Cis Dipole dipole Trans ... ... ... Cis dichloroethene Trans dichloroethene Melting Point/C -80 -50 Kink/ bend shape Cis dichloroethene Trans dichloroethene Boiling Point/C 60 48 Greater attraction Cis isomer – CI same side Bond polarity does not cancel Net dipole moment /POLAR Intermolecular forces stronger δ+ δ+ δ- δ- δ- ... ... Cis Trans Non polar Non polar Polar Polar Cis Trans …...... …...... ✓ ✓
- 14. RMM increases Number electron VDF increases H2O •2 hydrogen atoms •2 lone pairs on oxygen 4 Hydrogen bonding available Evidence for Hydrogen Bonding Boiling Point group 4 Hydrides Group 4, 5, 6, 7 Hydrides Group 4 – SiH4, GeH4, SnH4 Group 5 – PH3, AsH3, SbH3 Group 6 – H2S, H2Se, H2Te Group 7 – HCI, HBr, HI High boiling point for NH3, HF, H2O due to hydrogen bonding H2O HF HF •1 hydrogen atom •3 lone pairs on fluorine Lack hydrogen atoms for hydrogen bond NH3 NH3 •3 hydrogen atoms •1 lone pairs on ammonia Lack lone pairs for hydrogen bond
- 15. CH3-C=O CH3-C-CH3 CH3C-O-H CH3-O-CH3 CH3-N-H CH3-C-N-H I II II I II H O O H O Which of the following molecules are polar/non polar? ICI BCI3 CH2CI2 SF6 NF3 CO2 δ+ δ- δ+ δ- δ- δ- δ+ δ+ δ+ δ+ δ- δ- δ- δ- δ- δ- δ- δ- δ- δ- δ- δ- δ- Polar bond Polar Bond Polar Bond Polar Bond Polar Bond Polar Bond Bond Polarity Bond Polarity Bond Polarity Bond Polarity Bond Polarity Bond Polarity exist cancel exist cancel exist cancel (Asymmetric) (Symmetrical) (Asymmetrical) (Symmetrical) (Asymmetrical) (Symmetrical) ✓✓✓ Which of the following molecules have hydrogenbonding? CH3CHO CH3COCH3 CH3COOH CH3OCH3 CH3NH2 CH3CONH2 Hydrogen NOT No Hydrogen Bond Hydrogen attach No Hydrogen Bond Hydrogen attach Hydrogen attach attach to N,O,F to N, O, F to N, O, F to N, O, F ✕ ✕✓ ✓ ✓✕ ✕ ✕ ✕
